Insights into Calmodulin-Interacting Proteins
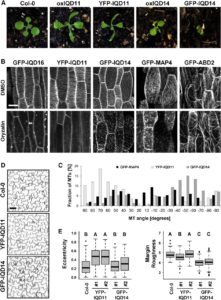 Calmodulin (CaM) and closely related CaM-like polypeptides are principal sensors of Ca2+ signals. The plant-specific IQ67 DOMAIN (IQD) family has emerged as possibly the largest class of CaM-interacting proteins with undefined molecular functions and biological roles. Bürstenbinder et al. (Plant Physiol. 173: 1692–1708) show that the 33 members of the IQD family in Arabidopsis differentially localize to multiple and distinct subcellular sites, including microtubule (MT) arrays, plasma membrane subdomains, and nuclear compartments. Intriguingly, the various IQD-specific localization patterns coincide with the subcellular patterns of IQD-dependent recruitment of CaM, suggesting that the diverse IQD members sequester Ca2+-CaM signaling modules to specific subcellular sites for precise regulation of Ca2+-dependent processes. Because MT localization is a hallmark of most IQD family members, the authors quantitatively analyzed GFP-labeled MT arrays in Nicotiana benthamiana cells transiently expressing GFP-IQD fusions and observed IQD-specific MT patterns, which point to a role of IQDs in MT organization and dynamics. Indeed, misexpression of select IQD proteins in Arabidopsis altered cellular MT orientation, cell shape, and organ morphology. Because IQDs share biochemical properties with scaffold proteins, the authors propose that the IQD family provides an assortment of platform proteins for integrating CaM-dependent Ca2+ signaling at multiple cellular sites to regulate cell function, shape, and growth.
Calmodulin (CaM) and closely related CaM-like polypeptides are principal sensors of Ca2+ signals. The plant-specific IQ67 DOMAIN (IQD) family has emerged as possibly the largest class of CaM-interacting proteins with undefined molecular functions and biological roles. Bürstenbinder et al. (Plant Physiol. 173: 1692–1708) show that the 33 members of the IQD family in Arabidopsis differentially localize to multiple and distinct subcellular sites, including microtubule (MT) arrays, plasma membrane subdomains, and nuclear compartments. Intriguingly, the various IQD-specific localization patterns coincide with the subcellular patterns of IQD-dependent recruitment of CaM, suggesting that the diverse IQD members sequester Ca2+-CaM signaling modules to specific subcellular sites for precise regulation of Ca2+-dependent processes. Because MT localization is a hallmark of most IQD family members, the authors quantitatively analyzed GFP-labeled MT arrays in Nicotiana benthamiana cells transiently expressing GFP-IQD fusions and observed IQD-specific MT patterns, which point to a role of IQDs in MT organization and dynamics. Indeed, misexpression of select IQD proteins in Arabidopsis altered cellular MT orientation, cell shape, and organ morphology. Because IQDs share biochemical properties with scaffold proteins, the authors propose that the IQD family provides an assortment of platform proteins for integrating CaM-dependent Ca2+ signaling at multiple cellular sites to regulate cell function, shape, and growth.







Leave a Reply
Want to join the discussion?Feel free to contribute!