HY5 and TZP cooperate to help plants respond to far-red light
By Cong Li, Lijuan Qi and Jigang Li
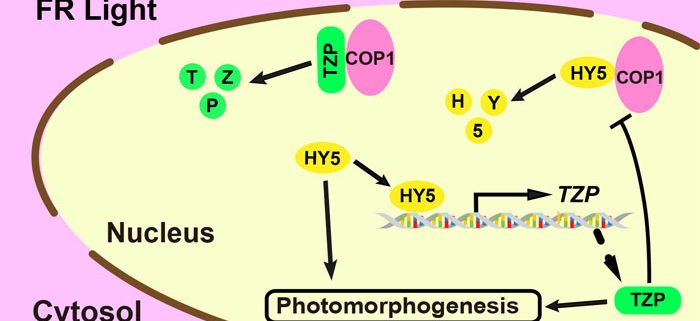
Background: Phytochrome A (phyA) is the far-red (FR) light photoreceptor in plants that is essential for initiating light responses under FR-rich environments, such as canopy shade. Upon FR light irradiation, phyA changes its conformation, translocates from the cytosol into the nucleus, and promotes the abundance of ELONGATED HYPOCOTYL5 (HY5). HY5 is a bZIP-family transcription factor that plays a critical role in transmitting the FR light signal by directly modulating the transcription of a large number of light-responsive genes. CONSTITUTIVE PHOTOMORPHOGENIC1 (COP1) is an E3 ubiquitin ligase that directly targets HY5 for degradation. We recently identified two mutant alleles of TANDEM ZINC-FINGER/PLUS3 (TZP) by forward genetic screening for mutants showing long hypocotyls in FR light and demonstrated that TZP acts as a key positive regulator of phyA signal transduction.
Question: How is TZP integrated into the phyA signaling network?
Findings: We uncovered that HY5 directly binds to a G-box motif in the TZP promoter and activates TZP transcription upon FR light exposure. Consequently, HY5 positively regulates TZP protein accumulation in FR light. In turn, TZP competes with HY5 to interact with COP1, and post-translationally promotes HY5 protein stability in FR light. However, TZP itself is also destabilized by COP1 in FR light, indicating that TZP promotes HY5 stability at the expense of being tagged for degradation by COP1. Collectively, our data demonstrate that HY5 and TZP mutually upregulate each other by distinct mechanisms in transmitting the FR light signal. In addition to their interdependent functions, tzp hy5 double mutants display an additive phenotype relative to their respective single mutants under high FR light intensities, indicating that TZP and HY5 also function in independent pathways.
Next steps: We aim to explore the detailed molecular mechanism underlying COP1-mediated degradation of TZP. In addition, since the valine-proline (VP) motif of TZP is not responsible for interacting with COP1, we will determine the critical amino acids of TZP that mediate TZP interaction with COP1.
Cong Li, Lijuan Qi, Shaoman Zhang, Xiaojing Dong, Yanjun Jing, Jinkui Cheng, Ziyi Feng, Jing Peng, Hong Li, Yangyang Zhou, Xiaoji Wang, Run Han, Jie Duan, William Terzaghi, Rongcheng Lin and Jigang Li. (2019). Mutual upregulation of HY5 and TZP in mediating phytochrome A signaling. Plant Cell. https://doi.org/10.1093/plcell/koab254