How plants keep their microbiota healthy (Nature)
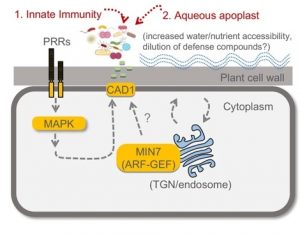 The large apoplastic intercellular space of plant leaves creates nutrient-rich niches for microbial colonization. To date, whether and how plants control the composition of leaf microbiota is poorly understood. Chen et al. reported that the Arabidopsis quadruple mutant (min7fls2efrcerk1 or mfec) defective in pattern-triggered immunity and a vesicle trafficking pathway (MIN7 encodes an ARF-GEF protein) showed tissue damage phenotypes when grown in soils. The mutant plants showed marked changes in the leaf endophytic bacterial community: 1) an overall increased bacterial population size, 2) decreased community diversity, and 3) a shift from a Firmicutes-rich community to a Proteobacteria-rich community. The authors demonstrated that the altered bacterial community is causal for leaf tissue damage phenotypes. Pairwise bacteria-bacteria competition assay in vitro and in vivo suggested that some Proteobacteria can outcompete most Firmicutes when they are in close proximity. Thus, in the mfec mutant, an increased abundance of leaf endophytic bacteria may stimulate microbe-microbe interactions, contributing to the conversion of a Firmicutes-rich community to a Proteobacteria-rich community. Finally, the authors found a point mutation in CONSTITUTIVELY ACTIVATED CELL DEATH1 (CAD1) gene alone phenocopied the mfec mutant, raising a possibility that CAD1 is a convergent component downstream of pattern-triggered immunity and the MIN7 vesicle-trafficking pathway. The results shown in this study resemble animal dysbiosis, a condition where altered microbiota negatively impacts the host health. This study reveals that the plant pattern-triggered immunity and MIN7 vesicle trafficking pathways have non-redundant and essential roles in preventing dysbiosis and provides insights into underlying molecular mechanisms. (Summary by Tatsuya Nobori @nobolly) Nature 10.1038/s41586-020-2185-0
The large apoplastic intercellular space of plant leaves creates nutrient-rich niches for microbial colonization. To date, whether and how plants control the composition of leaf microbiota is poorly understood. Chen et al. reported that the Arabidopsis quadruple mutant (min7fls2efrcerk1 or mfec) defective in pattern-triggered immunity and a vesicle trafficking pathway (MIN7 encodes an ARF-GEF protein) showed tissue damage phenotypes when grown in soils. The mutant plants showed marked changes in the leaf endophytic bacterial community: 1) an overall increased bacterial population size, 2) decreased community diversity, and 3) a shift from a Firmicutes-rich community to a Proteobacteria-rich community. The authors demonstrated that the altered bacterial community is causal for leaf tissue damage phenotypes. Pairwise bacteria-bacteria competition assay in vitro and in vivo suggested that some Proteobacteria can outcompete most Firmicutes when they are in close proximity. Thus, in the mfec mutant, an increased abundance of leaf endophytic bacteria may stimulate microbe-microbe interactions, contributing to the conversion of a Firmicutes-rich community to a Proteobacteria-rich community. Finally, the authors found a point mutation in CONSTITUTIVELY ACTIVATED CELL DEATH1 (CAD1) gene alone phenocopied the mfec mutant, raising a possibility that CAD1 is a convergent component downstream of pattern-triggered immunity and the MIN7 vesicle-trafficking pathway. The results shown in this study resemble animal dysbiosis, a condition where altered microbiota negatively impacts the host health. This study reveals that the plant pattern-triggered immunity and MIN7 vesicle trafficking pathways have non-redundant and essential roles in preventing dysbiosis and provides insights into underlying molecular mechanisms. (Summary by Tatsuya Nobori @nobolly) Nature 10.1038/s41586-020-2185-0