How COR27 and COR28 Promote Hypocotyl Growth: Bind to COP1 And Suppress HY5 Activity
Seedling growth and development rely on the successful integration of information from external signals, such as light and temperature, and endogenous processes, such as the rhythmic ticking of the circadian clock. Together, these signaling pathways tune hypocotyl elongation to allow seedlings to escape from the dark soil and reach the bright soil surface, followed by cotyledon expansion and suppression of hypocotyl growth during photomorphogenesis in the light.
A key signaling hub that regulates photomorphogenesis is the E3 ubiquitin ligase CONSTITUTIVE PHOTOMORPHOGENIC 1 (COP1) that targets proteins for degradation via the 26S proteasome in the dark, thereby controlling the levels of light signaling proteins (Lau and Deng, 2012). Conversely, light suppresses the activity of COP1 and its partner, SUPPRESSOR OF PHYTOCHROME A 1 (SPA1) via multiple regulatory mechanisms, leading to changes in downstream gene expression and appropriate developmental responses.
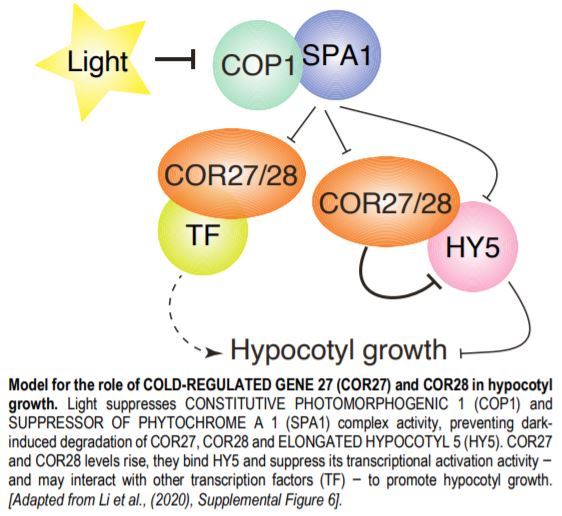 Light, temperature and clock signaling pathways share several common nodes. For example, both low temperatures and light regulate the expression of COLD REGULATED GENE 27 and 28 (COR27 and COR28). Likewise, COR27 and COR28 modulate the pace of the circadian clock (Li et al., 2016). In the current issue, Li et al. (2020) and Zhu et al. (2020) identify a new role for COR27 and COR28 in promoting hypocotyl growth via their interaction with the COP1-SPA1 complex and regulation of downstream gene expression.
Light, temperature and clock signaling pathways share several common nodes. For example, both low temperatures and light regulate the expression of COLD REGULATED GENE 27 and 28 (COR27 and COR28). Likewise, COR27 and COR28 modulate the pace of the circadian clock (Li et al., 2016). In the current issue, Li et al. (2020) and Zhu et al. (2020) identify a new role for COR27 and COR28 in promoting hypocotyl growth via their interaction with the COP1-SPA1 complex and regulation of downstream gene expression.
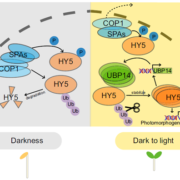
The two independent studies started with yeast two-hybrid screens to identify interaction partners for COR27 and COR28 (Li et al., 2020) or COP1 (Zhu et al., 2020). Both discovered that COR27 and COR28 interacted with COP1 and its partner, SPA1. As the COP1-SPA1 complex is involved in proteasomal degradation, both sets of authors measured COR27 protein levels in light- and dark-grown seedlings, in wild type and in weak cop1 mutants. COR27 accumulated in the light but was degraded in the dark, in a COP1- and 26S proteasome-dependent manner.
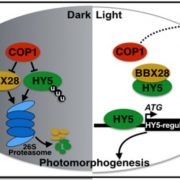
Analysis of the role of COR27 and COR28 during photomorphogenesis revealed their contributions to hypocotyl elongation: cor27 and cor27 cor28 mutants had shorter hypocotyls, while seedlings overexpressing COR27 or COR28 had longer hypocotyls in all light conditions, defining COR27 and COR28 as negative regulators of light signaling. As ELONGATED HYPOCOTYL 5 (HY5) is a major transcriptional regulator of light responses that functions directly downstream of the COP1-SPA1 complex, both sets of authors then tested whether COR27 interacted with HY5; indeed they do. COR27 inhibited HY5 binding to DNA, thereby suppressing its transcriptional activation activity (Zhu et al., 2020) and highlighting a mechanism by which COR27 and COR28 may affect hypocotyl elongation (see Figure). COR27 may also exhibit additional functions in hypocotyl growth besides suppressing HY5 activity, based on hypocotyl length differences between the hy5 mutant and seedlings overexpressing or lacking COR27 in the hy5 background.
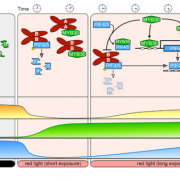
Another master regulator of light signaling and hypocotyl elongation is the bHLH transcription factor PHYTOCHROME-INTERACTING FACTOR 4 (PIF4). Both studies set out to test whether impairment of COR27 function affected PIF4 mRNA levels. Zhu et al. (2020) detected reduced PIF4 mRNA levels in the cor27 mutant in the afternoon, but not at other time-points over the course of a 12 h light/12 h dark diurnal cycle. By contrast, Li et al. (2020) observed lower PIF4 expression in the cor27 cor28 mutant at night in seedlings grown in short days (8 h light/ 16 h dark), but did not test other time-points. Addressing the role of photoperiod and light intensity may help clarify the interactions between COR27, COR28 and PIF4 expression.
The studies by Li et al. (2020) and Zhu et al. (2020) present strong evidence for a COP1-dependent role of COR27 and COR28 in the promotion of hypocotyl growth. How this effect is executed through downstream targets, such as HY5 and potentially other transcription factors, will be the next pieces to place in the puzzle of temperature, light and circadian clock signaling.
Hanna Hõrak
Institute of Technology
University of Tartu, Estonia
ORCID: 0000-0002-6392-859X
REFERENCES
Lau, O.S. and Deng, X.W. (2012). The photomorphogenic repressors COP1 and DET1: 20 years later. Trends Plant Sci. 17: 584–593.
Li, X., Ma, D., Lu, S.X., Hu, X., Huang, R., Liang, T., Xu, T., Tobin, E.M., and Liu, H. (2016). Blue Light- and Low Temperature-Regulated COR27 and COR28 Play Roles in the Arabidopsis Circadian Clock. Plant Cell 28: 2755–2769.
Li, X., Liu, C., Zhao, Z., Ma, D., Zhang, J., Yang, Y., Liu, Y., and Liu, H. (2020). COR27 and COR28 are Novel Regulators of the COP1–HY5 Regulatory Hub and Photomorphogenesis in Arabidopsis. Published August 2020. DOI: https://doi.org/10.1105/tpc.20.00195
Zhu, W., Zhou, H., Lin, F., Zhao, X., Jiang, Y., Xu, D., and Deng, XW. (2020). COLD-REGULATED GENE 27 integrates signals from light and the circadian rhythm to promote hypocotyl growth in Arabidopsis. Published July 2020. DOI: https://doi.org/10.1105/tpc.20.00192