Hold Me Closer: Meiotic Crossover Formation and FANCD2
Meiosis takes a single, diploid cell and turns it into four haploid spores. The equal distribution of genetic material is critical for genome stability across generations, and relies heavily on proper pairing of chromosomes and their timely release. During the first meiotic division, crossovers (COs) are responsible both for the exchange of genetic material between homologues and their stable association until segregation. Herein lies the problem: COs depend on double stranded breaks (DSBs) along genomic DNA. By necessity, meiosis is thus a choreographed dance between controlled DNA damage and repair. In a new study, Kurzbauer and colleagues explain how the Arabidopsis FANCD2 protein contributes to genome stability by promoting CO formation.
The FANCD2 gene gets its name from Fanconi Anemia, a human autosomal recessive disorder characterized by chromosome instability, predisposition to cancer and reduced fertility. Several FA genes are conserved across higher eukaryotes, including the worm Caenorabditis elegans and Arabidopsis (with a remarkable 44% similarity to their human counterpart). In contrast to mammals, C. elegans and Arabidopsis fancd2 mutants are viable and fertile (Collis et al. 2006; Kurzbauer et al., 2018), making them great genetic models. Arabidopsis fancd2 mutants do produce fewer seeds per silique, potentially pointing to a problem during meiosis.
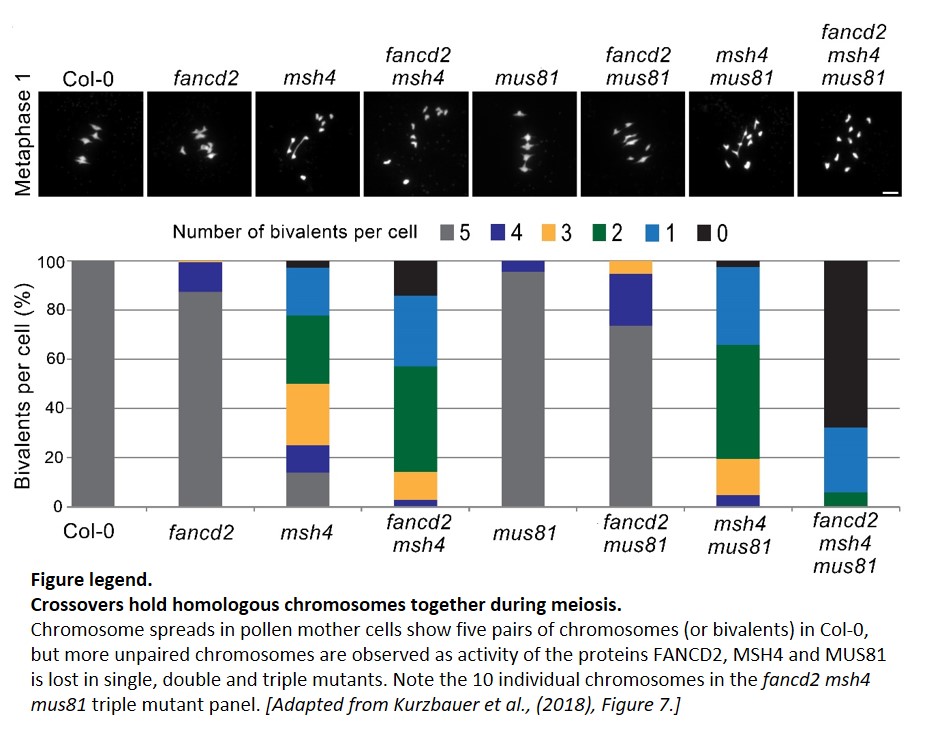
Using a combination of cytological and genetic analyses, the authors determined the position of FANCD2 during Arabidopsis meiosis. First, they observed that fancd2 mutants accumulate 14% fewer COs. A reduction in CO number during meiosis will result in chromosome missegregation, as homologues can no longer be held together. Such unpaired chromosomes, or univalents, are observed in 13% of fancd2 pollen mother cells by metaphase I. Second, FANCD2 genetically interacts with ATM and ATR, encoding two protein kinases that may phosphorylate FANCD2. atm mutants exhibit chromosome fragmentation, and this phenotype is enhanced in atm fancd2 double mutants. Chromosome fragmentation is caused by the formation of DSBs that are not repaired into COs. Indeed, loss of the transesterase SPO11 (responsible for the formation of DSBs) completely rescues fragmentation, but now all chromosomes are present as univalents during metaphase I because of a global loss of COs. These results therefore place FANCD2 downstream of SPO11-catalyzed DSBs formation. Next, the authors show that the number of RAD51 foci along chromosomes is normal in fancd2 mutants. RAD51 marks meiotic recombination spots formed after DSBs, but before CO formation. In short: DSBs form normally in fancd2, and DNA repair enzymes are recruited at the right place and the right time.
Does FANCD2 contribute to the formation of COs directly? Two types of COs can form during meiosis: one type of CO prevents the formation of another CO close by (these are called interference-sensitive), while COs from the other, interference-insensitive type, are sprinkled randomly along chromosomes. Loss of fancd2 maintains interference but genetic distances are slightly shorter, in agreement with fewer COs overall. A more detailed genetic analysis places FANCD2 in a pathway parallel to both interference-sensitive COs (involving the protein MSH4) and interference-insensitive COs (promoted by the protein MUS81), which has not been observed in animal systems to date. fancd2 msh4 mus81 triple mutants form very few COs, and as a result most chromosomes are present as unpaired univalents (see Figure).
The following model emerges from this research: FANCD2 acts after DSB formation and may recruit nucleases to DNA lesion sites to resolve them into COs. Questions that remain include what are the factors that bring FANCD2 to DSB sites, and what proteins FANCD2 in turn recruit to promote CO formation. In addition, the role of other conserved FA genes (Girard et al., 2014) should be reassessed with selected double and triple mutant combinations with msh4, mus81 and spo11.
REFERENCES
Collis SJ, Barber LJ, Ward JD, Martin JS and Boulton SJ (2006).C. elegans FANCD2 responds to replication stress and functions in interstrand cross-link repair. DNA REPAIR 5: 1398–1406.
Girard C, Crismani W, Froger N, Mazel J, Lemhemdi A, Horlow C, and Mercier R (2014). FANCM-associated proteins MHF1 and MHF2, but not the other Fanconi anemia factors, limit meiotic crossovers. Nucleic Acids Res. 42: 9087–9095.
Kurzbauer M-T, Pradillo M, Kerzendorfer C, Sims J, Ladurner R, Oliver C, Janisiw MP, Mosiolek M, Schweizer D, Copenhaver GP and Schlögelhofer P (2018). Arabidopsis thaliana FANCD2 Promotes Meiotic Crossover Formation. Published January 2018, DOI: https://doi.org/10.1105/tpc.17.00745