Functionally overlapping but molecularly distinct TGN subdomains of two Epsin-like proteins (PNAS)
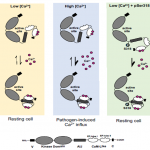
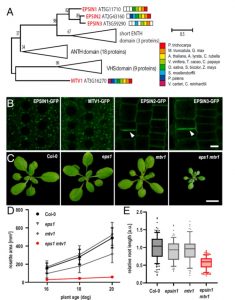 The trans-Golgi networks (TGN) is a major protein sorting station involved in trafficking or recycling of endosomal materials. This busiest hub in the cell has at least four important pathways including the anterograde routes of secretory, recycling, vacuolar transport and retrograde endocytic pathways. At molecular level, it remains unclear how sorting of cargo and distribution into different vesicles are controlled. Heinze et al. identified two Arabidopsis Epsin-like proteins, EPSIN1 and MTV1, localized in speckles resembling endosomal structures at the TGN. The single mutants of these genes do not show drastic phenotypes but the double mutant eps1mtv1 exhibited dwarf phenotype, suggesting their functional redundancy. EPSIN1 and MTV1 contribute to the vacuolar transport and a subset of secretory cargo independently of the TGN protein ECHIDNA. However, these two proteins are not involved endocytic or recycling pathways. Interestingly, EPSIN1 and MTV1 do not colocalize and a large distance was revealed between them, indicating their presence at two distinct TGN subdomains. EPSIN1 interacts and colocalizes with AP-1 (Adaptor protein), whereas MTV1 acts in the same pathway as AP-4 and is recruited by it. Overall, these two Epsin-like proteins are phenotypically and functionally overlapping, but the subcellular localization and molecular interaction are distinct. (Summary by Min May Wong @wongminmay) PNAS 10.1073/pnas.2004822117
The trans-Golgi networks (TGN) is a major protein sorting station involved in trafficking or recycling of endosomal materials. This busiest hub in the cell has at least four important pathways including the anterograde routes of secretory, recycling, vacuolar transport and retrograde endocytic pathways. At molecular level, it remains unclear how sorting of cargo and distribution into different vesicles are controlled. Heinze et al. identified two Arabidopsis Epsin-like proteins, EPSIN1 and MTV1, localized in speckles resembling endosomal structures at the TGN. The single mutants of these genes do not show drastic phenotypes but the double mutant eps1mtv1 exhibited dwarf phenotype, suggesting their functional redundancy. EPSIN1 and MTV1 contribute to the vacuolar transport and a subset of secretory cargo independently of the TGN protein ECHIDNA. However, these two proteins are not involved endocytic or recycling pathways. Interestingly, EPSIN1 and MTV1 do not colocalize and a large distance was revealed between them, indicating their presence at two distinct TGN subdomains. EPSIN1 interacts and colocalizes with AP-1 (Adaptor protein), whereas MTV1 acts in the same pathway as AP-4 and is recruited by it. Overall, these two Epsin-like proteins are phenotypically and functionally overlapping, but the subcellular localization and molecular interaction are distinct. (Summary by Min May Wong @wongminmay) PNAS 10.1073/pnas.2004822117