FERONIA-RALF and G Proteins in Guard Cell Response
 Heterotrimeric guanine nucleotide-binding (G) proteins are composed of Ga, Gb, and Gg subunits and function as molecular switches in signal transduction. In Arabidopsis, there is one canonical Ga (GPA1), three extra-large Ga (XLG1, XLG2, and XLG3), one Gb (AGB1), and three Gg (AGG1 to 3) subunits. Despite the limited types of G protein subunits in Arabidopsis, G proteins have been shown to participate in numerous processes related to plant development and organ morphogenesis, cell wall composition, hormone responses, responses to
light stimuli,
and biotic and abiotic stresses. For example,
both GPA1 and AGB1 play positive roles in aspects of abscisic acid (ABA)-mediated regulation of stomatal apertures. Both gpa1 and agb1 mutants are hypo-sensitive to ABA inhibition of stomatal opening but
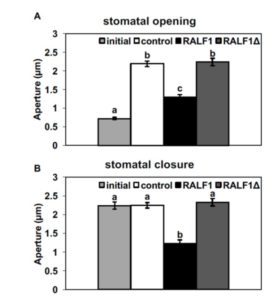
show wild-type responses to ABA promotion of stomatal closure. To shed further light on AGB1 signal transduction, Yu et al. (10.1104/pp.17.01277) studied plasma membrane-enriched proteins using immunoprecipitation followed by mass spectrometry to identify the protein interactors of AGB1. After eliminating proteins present in the control immunoprecipitation, commonly identified contaminants, and organellar proteins, a total of 103 candidate AGB1-associated proteins were identified. The authors confirm the physical interaction between AGB1 and the receptor-like kinase FERONIA (FER) using bimolecular fluorescence complementation. Previous studies have shown that the Rapid Alkalinization Factor (RALF) family of polypeptides are ligands of FER. The authors now demonstrate that RALF1 regulates stomatal apertures and does so in a G protein-dependent manner, inhibiting stomatal opening and promoting stomatal closure in wild-type but not in agb1 mutants. They further show that AGGs and XLGs, but not the canonical Ga, GPA1, participate in RALF1-mediated stomatal signaling. These results suggest that FER acts as a G protein-coupled receptor for plant heterotrimeric G proteins.
Heterotrimeric guanine nucleotide-binding (G) proteins are composed of Ga, Gb, and Gg subunits and function as molecular switches in signal transduction. In Arabidopsis, there is one canonical Ga (GPA1), three extra-large Ga (XLG1, XLG2, and XLG3), one Gb (AGB1), and three Gg (AGG1 to 3) subunits. Despite the limited types of G protein subunits in Arabidopsis, G proteins have been shown to participate in numerous processes related to plant development and organ morphogenesis, cell wall composition, hormone responses, responses to
light stimuli,
and biotic and abiotic stresses. For example,
both GPA1 and AGB1 play positive roles in aspects of abscisic acid (ABA)-mediated regulation of stomatal apertures. Both gpa1 and agb1 mutants are hypo-sensitive to ABA inhibition of stomatal opening but
show wild-type responses to ABA promotion of stomatal closure. To shed further light on AGB1 signal transduction, Yu et al. (10.1104/pp.17.01277) studied plasma membrane-enriched proteins using immunoprecipitation followed by mass spectrometry to identify the protein interactors of AGB1. After eliminating proteins present in the control immunoprecipitation, commonly identified contaminants, and organellar proteins, a total of 103 candidate AGB1-associated proteins were identified. The authors confirm the physical interaction between AGB1 and the receptor-like kinase FERONIA (FER) using bimolecular fluorescence complementation. Previous studies have shown that the Rapid Alkalinization Factor (RALF) family of polypeptides are ligands of FER. The authors now demonstrate that RALF1 regulates stomatal apertures and does so in a G protein-dependent manner, inhibiting stomatal opening and promoting stomatal closure in wild-type but not in agb1 mutants. They further show that AGGs and XLGs, but not the canonical Ga, GPA1, participate in RALF1-mediated stomatal signaling. These results suggest that FER acts as a G protein-coupled receptor for plant heterotrimeric G proteins.