Dark-Induced Nuclear Positioning in Leaf Cells
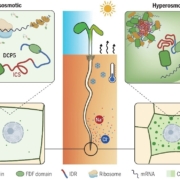
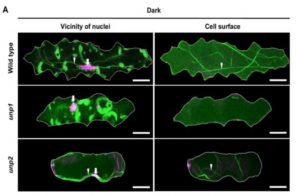 The appropriate spatial arrangement of nuclei is essential for various cellular activities during cell division, growth, migration, and differentiation in eukaryotes. In plants, nuclear positioning is also required for proper responses to environmental stimuli, including pathogen infection, touch, temperature, and light. The nuclei of Arabidopsis (Arabidopsis thaliana) leaf cells move to the anticlinal walls of cells in response to strong ultraviolet-A light. This response mitigates the DNA damage and cell death caused by ultraviolet irradiation. Light-induced nuclear positioning depends on chloroplast movement. Nuclei cannot move by themselves; instead, nucleus-attached chloroplasts carry the nuclei to anticlinal walls of cells. Light-induced chloroplast movement is a well-known process that is regulated by the blue light-receptor phototropins, actin filaments, and several regulatory proteins. Hence, in mutants lacking these proteins, this type of nuclear positioning does not occur. Unlike light-induced nuclear positioning, the mechanism underlying dark-induced nuclear positioning is not fully understood. Iwabuchi et al. (10.1104/pp.18.01150) show that ANGUSTIFOLIA (AN) and ACTIN7 regulate centripetal nuclear positioning in Arabidopsis (Arabidopsis thaliana) leaves. Two mutants defective in the positioning of nuclei in the dark were isolated and designated as unusual nuclear positioning 1 (unp1) and unp2. In the dark, nuclei of unp1 were positioned at the anticlinal walls of adaxial and abaxial mesophyll cells and abaxial pavement cells, whereas the nuclei of unp2 were positioned at the anticlinal walls of mesophyll and pavement cells in both the adaxial and abaxial sides. unp1 was caused by a dominant-negative mutation in ACTIN7, and unp2 resulted from a recessive mutation in AN. Actin filaments in unp1 were fragmented and reduced in number, which led to pleiotropic defects in nuclear morphology, cytoplasmic streaming, and plant growth. The mutation in AN caused aberrant positioning of nuclei-associated actin filaments at the anticlinal walls. AN also physically interacts with plant-specific dual-specificity tyrosine phosphorylation-regulated kinases (DYRKPs). The results presented suggest that the AN-DYRKP complex regulates the alignment of actin filaments during centripetal nuclear positioning in leaf cells.
The appropriate spatial arrangement of nuclei is essential for various cellular activities during cell division, growth, migration, and differentiation in eukaryotes. In plants, nuclear positioning is also required for proper responses to environmental stimuli, including pathogen infection, touch, temperature, and light. The nuclei of Arabidopsis (Arabidopsis thaliana) leaf cells move to the anticlinal walls of cells in response to strong ultraviolet-A light. This response mitigates the DNA damage and cell death caused by ultraviolet irradiation. Light-induced nuclear positioning depends on chloroplast movement. Nuclei cannot move by themselves; instead, nucleus-attached chloroplasts carry the nuclei to anticlinal walls of cells. Light-induced chloroplast movement is a well-known process that is regulated by the blue light-receptor phototropins, actin filaments, and several regulatory proteins. Hence, in mutants lacking these proteins, this type of nuclear positioning does not occur. Unlike light-induced nuclear positioning, the mechanism underlying dark-induced nuclear positioning is not fully understood. Iwabuchi et al. (10.1104/pp.18.01150) show that ANGUSTIFOLIA (AN) and ACTIN7 regulate centripetal nuclear positioning in Arabidopsis (Arabidopsis thaliana) leaves. Two mutants defective in the positioning of nuclei in the dark were isolated and designated as unusual nuclear positioning 1 (unp1) and unp2. In the dark, nuclei of unp1 were positioned at the anticlinal walls of adaxial and abaxial mesophyll cells and abaxial pavement cells, whereas the nuclei of unp2 were positioned at the anticlinal walls of mesophyll and pavement cells in both the adaxial and abaxial sides. unp1 was caused by a dominant-negative mutation in ACTIN7, and unp2 resulted from a recessive mutation in AN. Actin filaments in unp1 were fragmented and reduced in number, which led to pleiotropic defects in nuclear morphology, cytoplasmic streaming, and plant growth. The mutation in AN caused aberrant positioning of nuclei-associated actin filaments at the anticlinal walls. AN also physically interacts with plant-specific dual-specificity tyrosine phosphorylation-regulated kinases (DYRKPs). The results presented suggest that the AN-DYRKP complex regulates the alignment of actin filaments during centripetal nuclear positioning in leaf cells.