CPK32 regulates cellulose biosynthesis through post-translational modification of cellulose synthase
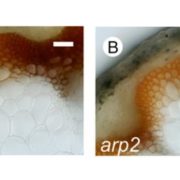
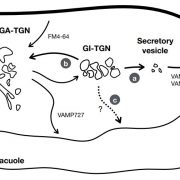
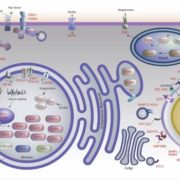
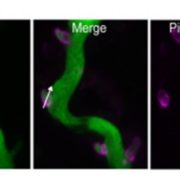
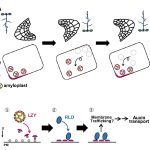
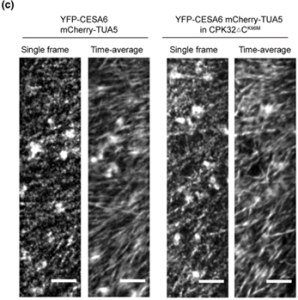 Cellulose in one of the most abundant polymers on the planet and is synthesized by plasma membrane-bound cellulose synthases (CESAs). Phosphorylation plays a role in CESA regulation, however the kinases which catalyse the phosphorylation are not well described. Here Xin et al. identified calcium-dependent protein kinase 32 (CPK32) as an interactor of CESA3 in a yeast two hybrid screen. In vitro kinase assays revealed that CPK32 phosphorylates the catalytic domain of CESA3. To investigate how CPK32 phosphorylation affects CESA activity, they mutated a lysine residue in the CPK32 ATP-binding site (CPK32ΔCK96M). This mutation prevented kinase activity but not the interaction with CESAs. When CPK32ΔCK96M was transformed into Arabidopsis thaliana plants expressing YFP-tagged CESAs, CESA-YFP movement decreased by 28%. There was also a decrease in CESA-YFP stability. Protein degradation experiments showed a 30% reduction in the amount of CESA-YFP when CPK32ΔCK96M was expressed. This suggests a novel role of phosphorylation in controlling CESA stability during cellulose biosynthesis. (Summary by Rose McNelly @Rose_McN) New Phytol. 10.1111/nph.19106
Cellulose in one of the most abundant polymers on the planet and is synthesized by plasma membrane-bound cellulose synthases (CESAs). Phosphorylation plays a role in CESA regulation, however the kinases which catalyse the phosphorylation are not well described. Here Xin et al. identified calcium-dependent protein kinase 32 (CPK32) as an interactor of CESA3 in a yeast two hybrid screen. In vitro kinase assays revealed that CPK32 phosphorylates the catalytic domain of CESA3. To investigate how CPK32 phosphorylation affects CESA activity, they mutated a lysine residue in the CPK32 ATP-binding site (CPK32ΔCK96M). This mutation prevented kinase activity but not the interaction with CESAs. When CPK32ΔCK96M was transformed into Arabidopsis thaliana plants expressing YFP-tagged CESAs, CESA-YFP movement decreased by 28%. There was also a decrease in CESA-YFP stability. Protein degradation experiments showed a 30% reduction in the amount of CESA-YFP when CPK32ΔCK96M was expressed. This suggests a novel role of phosphorylation in controlling CESA stability during cellulose biosynthesis. (Summary by Rose McNelly @Rose_McN) New Phytol. 10.1111/nph.19106