Chloroplast proteostasis prevents aggregation of Huntington’s disease-causing human polyQ protein
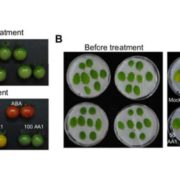
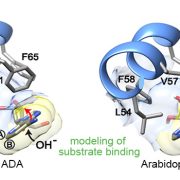
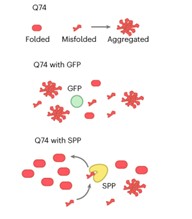 Certain human neurodegenerative disorders are caused by aggregation of disordered proteins. In particular, Huntington’s disease is caused by aggregation of a protein called huntingtin, which contains long stretches of glutamine (Q). Llama et al. observed that proteins with long stretches of glutamine are also present in plants, but they do not seem to cause disease. To investigate how plants control aggregation of these polyQ-containing proteins, the authors generated transgenic Arabidopsis thaliana expressing a fragment of human huntingtin labelled with a fluorophore. Using confocal microscopy, they observed that huntingtin remains soluble in plant cells in normal growth conditions but becomes aggregated under heat stress. They found that the expressed protein interacts with components of chloroplast protein import and chloroplast proteases, and that impairment of these chloroplast functions caused aggregation of huntingtin in plant cells. Thus, the authors concluded that plants use chloroplast proteostasis to control aggregation of polyglutamine proteins. Expression of a chloroplast protease, the chloroplast stromal processing peptidase (SPP), can also prevent aggregation of huntingtin both in a human cell line and in nematodes. This work presents plant chloroplast proteostasis as a novel path towards developing treatments for human neurodegenerative diseases. (Summary by Ángel Vergara @ngelVerCru) ) Nature Aging 10.1038/s43587-023-00502-1
Certain human neurodegenerative disorders are caused by aggregation of disordered proteins. In particular, Huntington’s disease is caused by aggregation of a protein called huntingtin, which contains long stretches of glutamine (Q). Llama et al. observed that proteins with long stretches of glutamine are also present in plants, but they do not seem to cause disease. To investigate how plants control aggregation of these polyQ-containing proteins, the authors generated transgenic Arabidopsis thaliana expressing a fragment of human huntingtin labelled with a fluorophore. Using confocal microscopy, they observed that huntingtin remains soluble in plant cells in normal growth conditions but becomes aggregated under heat stress. They found that the expressed protein interacts with components of chloroplast protein import and chloroplast proteases, and that impairment of these chloroplast functions caused aggregation of huntingtin in plant cells. Thus, the authors concluded that plants use chloroplast proteostasis to control aggregation of polyglutamine proteins. Expression of a chloroplast protease, the chloroplast stromal processing peptidase (SPP), can also prevent aggregation of huntingtin both in a human cell line and in nematodes. This work presents plant chloroplast proteostasis as a novel path towards developing treatments for human neurodegenerative diseases. (Summary by Ángel Vergara @ngelVerCru) ) Nature Aging 10.1038/s43587-023-00502-1