Cauliflower mosaic virus uses Arabidopsis RNA processing body components to its advantage
By Gesa Hoffmann and Anders Hafrén, Swedish University of Agricultural Sciences
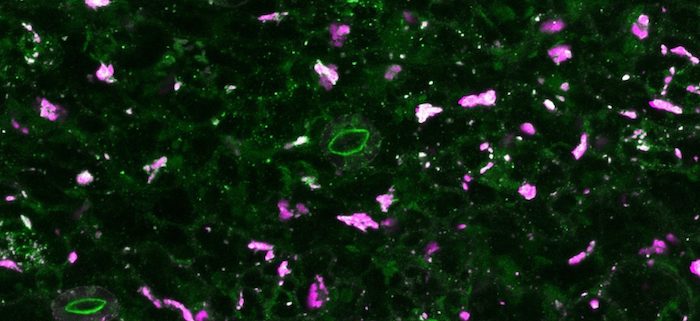
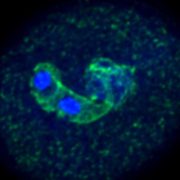
Background: Viruses are unique in their ability to reuse and recycle host proteins and other components for their own benefit. Cauliflower mosaic virus (CaMV) forms special structures inside the host cells known as viral factories to facilitate efficient replication and escape defense. Viral factories consist of viral proteins, as well as particles and nucleic acids, but also numerous host proteins and ribosomes that are co-opted into these structures. Building on knowledge from the animal field, RNA granules, including stress granules and processing bodies, are at the forefront of viral disease regulation. Several granule-localized proteins directly interact and influence virus replication.
Question: We investigated the role of processing body components in CaMV infection. We wanted to elucidate the interplay from two sides: What is the effect of CaMV infection on the localization and abundance of processing body components, but also how do these proteins influence CaMV replication and especially viral protein production?
Findings: Decapping proteins DCP5 and LSM1 localize to viral factories during CaMV infection. CaMV DNA and protein accumulation, but not RNA levels, are reduced in Arabidopsis dcp5 and lsm1 mutants. We found that viral RNA is not a target of LSM1-mediated decapping and that RNA stability is not affected in either mutant. We examined dcp5 and lsm1 single mutants as well as double mutants with RNA dependent RNA polymerase 6 (rdr6), finding that less viral RNA was associated with ribosomes in the single but not double mutants. Thus, processing body proteins help the virus evade translational repression by RDR6.
Next steps: We do not yet know how RDR6 mediates translational repression of viral RNA in the absence of DCP5 or LSM1. Elucidating the exact mechanism and which roles the viral factory and viral proteins play in this interaction will help further our understanding of plant virus infections.
Gesa Hoffmann, Amir Mahboubi, Heinrich Bente, Damien Garcia, Johannes Hanson, and Anders Hafrén (2022). Arabidopsis RNA processing body components LSM1 and DCP5 aid in the evasion of translational repression during Cauliflower mosaic virus infection. https://doi.org/10.1093/plcell/koac132