Open Access Shy Girl Gives Kiwifruit Male Flowers
From the human perspective, separate sexes are the norm while hermaphroditism is an exotic concept. For plants, hermaphroditism is the norm. Dioecy, separate male and female individuals, is rare and dispersed in the angiosperm phylogeny (Käfer et al 2017, Renner 2014). In fact, dioecy is rare enough that it was long believed to be an evolutionary dead end because it can reduce the likelihood of reproduction for these sessile organisms. Recent models suggest, to the contrary, that the evolution of dioecy may be reversible, with species transitioning in and out of dioecy at a certain frequency (Käfer et al. 2017), resulting in the current patchwork pattern along the phylogenetic tree.
The genetic changes responsible for transitions from hermaphroditism to dioecy remain largely unknown. Theory predicts that at least two mutations are necessary to evolve separate sexes. One must result in male sterility while the second must result in female sterility. Polyploidization or gene duplication events may facilitate the emergence of these mutations and the transition to dioecy. Non-recombining sex-determining regions have been identified in some dioecious plants including Silene latifolia, asparagus, papaya, and poplar, but the sex-determining genes and developmental processes responsible for separate sexes remain largely unidentified (but see Harkess et al. 2017). Further, since dioecy evolved independently many times in the angiosperm history (Renner, 2014), each transition to dioecy may involve different genes and evolutionary scenarios.
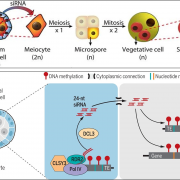
Akagi and coauthors (Akagi et al., 2018) tackled the evolution of dioecy in kiwifruit. The genus Actinidia hosts mostly dioecious species, suggesting that separate sex is the ancestral character. Sex-linked segments of the kiwifruit genome were identified through high coverage whole-genome sequencing of male and female individuals. These segments form a small male-specific 0.49Mb region within chromosome 25, flanked by recombining regions. This sex-determining area hosts sixty-one genes. From the pattern of genetic diversity in these candidate genes, the authors suggest that dioecy appeared in Actinidia 20 millions years ago, probably after a paleoploidization event.
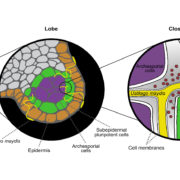
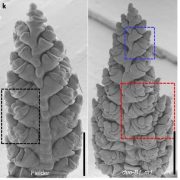
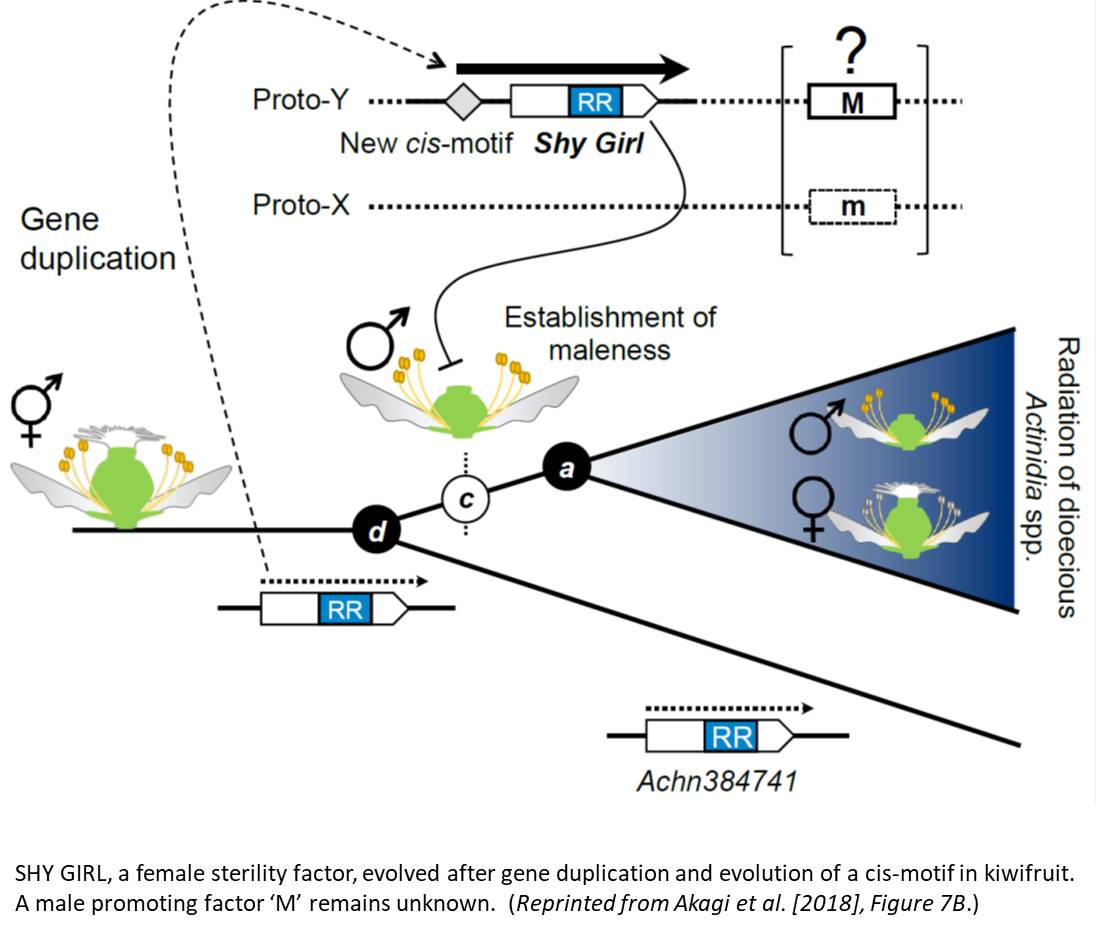
Morphologically, female kiwifruit flowers look like hermaphrodites but produce sterile pollen, while male flowers produce reduced carpels (see figure). The authors focused on identifying the genetic basis of female sterility. By analysing transcripts from young flower buds and carpels, the authors identified a male-specific type-C cytokinin response regulator expressed in developing flowers. This gene, named Shy Girl (SyGl), is expressed specifically at the surface of the rudimentary carpel in male flowers. When expressed in transgenic Arabidopsis or Nicotiana plants, this gene suppresses carpel development resulting in female sterility. The authors also show that application of synthetic cytokinin on the male rudimentary carpel partially restores female function in kiwifruit. Comparative gene expression analysis of SyGl and an autosomal sister gene reveal changes in the regulatory region while both genes kept the same protein function. This suggests the evolution of a cis-element in the SyGl promoter, conferring the specific expression pattern observed and the evolution of its function as a suppressor of female organ development in male flowers.
The gene responsible for pollen sterility in female flowers was not found in the current set of analyses. It requires transcriptome analyses from different tissues and flower development stage, the male organs, developing later than the female ones. Nonetheless, these findings add to an emerging mechanistic understanding of the evolution of sex determination genes, sex chromosomes, and dioecy in flowering plants.
REFERENCES
Akagi, T., Henry, I.M., Ohtani, H., Morimoto, Y., Beppu, K., Kataoka, I., and Tao R. (2018) A Y-encoded suppressor of feminization arose via lineage-specific duplication of a cytokinin response regulator in kiwifruit. Plant Cell https://doi.org/10.1105/tpc.17.00787
Harkess, A., Zhou, J., Xu, C., Bowers, J. E., Hulst, R., Ayyampalayam, S., … & Tang, H. (2017). The asparagus genome sheds light on the origin and evolution of a young Y chromosome. Nature Comm. 8: 1279.
Käfer, J., Marais, G. A., and Pannell, J. R. (2017). On the rarity of dioecy in flowering plants. Molec. Ecol. 26: 1225-1241.
Renner, S. S. (2014). The relative and absolute frequencies of angiosperm sexual systems: dioecy, monoecy, gynodioecy, and an updated online database. Amer. J. Bot. 101: 1588-1596.