Biosynthesis of the nitrogenase active-site cofactor precursor NifB-co in Saccharomyces cerevisiae (PNAS)
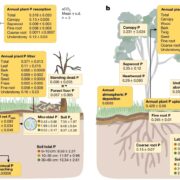
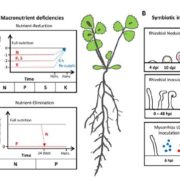
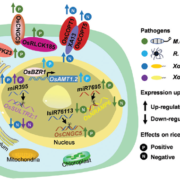
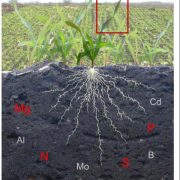
 Nitrogenases are large, multisubunit enzymes that require complex metal cofactors. They are the only enzymes capable of fixing N2 into usable form, and are only produced by some archaea and bacteria; “nitrogen-fixing” legumes actually depend on the presence of their bacterial symbionts for this function. Because applying nitrogenous fertilizers to crops is environmentally costly, many efforts are underway to develop nitrogen-fixing plants. One approach would be for the plant to plant produce nitrogenase itself, but this is proving to be a difficult challenge. Previous studies have suggested that the bacteria-derived mitochondria would be the optimal place for nitrogenase production, and many of the genes encoding nitrogenase subunits have been successfully expressed in yeast mitochondria, but the resulting complex lacked the essential metal cofactor. After an impressive amount of work and starting with 25 genes of bacterial and archaeal origin, Burén et al. have now succeeded in getting this cofactor synthesized in yeast mitochondria, paving the way for formation of the complete holoenzyme. A couple of key findings were that gene sequences did not predict successful protein expression, solubility and activity, and that the successful strategy involved expressing genes from the extreme thermophile Methanothermobacter thermautotrophicus, which might account for the proteins’ greater stability in a heterologous system. (Summary by Mary Williams) Proc. Natl. Acad. Sci. USA 10.1073/pnas.1904903116
Nitrogenases are large, multisubunit enzymes that require complex metal cofactors. They are the only enzymes capable of fixing N2 into usable form, and are only produced by some archaea and bacteria; “nitrogen-fixing” legumes actually depend on the presence of their bacterial symbionts for this function. Because applying nitrogenous fertilizers to crops is environmentally costly, many efforts are underway to develop nitrogen-fixing plants. One approach would be for the plant to plant produce nitrogenase itself, but this is proving to be a difficult challenge. Previous studies have suggested that the bacteria-derived mitochondria would be the optimal place for nitrogenase production, and many of the genes encoding nitrogenase subunits have been successfully expressed in yeast mitochondria, but the resulting complex lacked the essential metal cofactor. After an impressive amount of work and starting with 25 genes of bacterial and archaeal origin, Burén et al. have now succeeded in getting this cofactor synthesized in yeast mitochondria, paving the way for formation of the complete holoenzyme. A couple of key findings were that gene sequences did not predict successful protein expression, solubility and activity, and that the successful strategy involved expressing genes from the extreme thermophile Methanothermobacter thermautotrophicus, which might account for the proteins’ greater stability in a heterologous system. (Summary by Mary Williams) Proc. Natl. Acad. Sci. USA 10.1073/pnas.1904903116