Biosynthesis of Montbretin, an Anti-Diabetes Drug
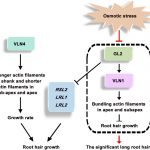
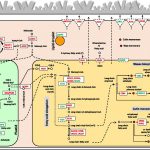
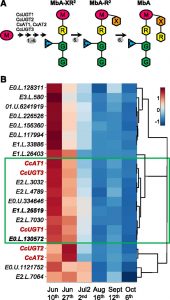 Diabetes and obesity are major health challenges. Diabetes alone affects over 422 million people worldwide and is among the top ten leading causes of death. Type-2 diabetes (T2D) is characterized by the body’s inefficient use of insulin, which leads to elevated blood Glc levels with detrimental effects on different organs and increased risk of dying prematurely. The plant metabolite montbretin A (MbA) is being developed as an anti-diabetes and anti-obesity treatment due to its potent and specific inhibition of the human pancreatic a-amylase. MbA is a complex acylated flavonol glycoside formed in small amounts in the corms of montbretia (Crocosmia X crocosmiiflora), a member of the iris family, during the early summer. Unfortunately. the spatial and temporal patterns of MbA accumulation limit its supply for drug development and application. Irmisch et al. (10.1104/pp.20.00522) are exploring MbA biosynthesis with the aim of enabling the metabolic engineering of this rare and valuable compound. Genes and enzymes for the first four steps of MbA biosynthesis, starting from the flavonol precursor myricetin, have recently been identified. Now, the authors describe the gene discovery and functional characterization of the remaining two enzymes of MbA biosynthesis. The authors reveal that two UDP-glycosyltransferases, CcUGT4 and CcUGT5, catalyze consecutive reactions in the formation of the disaccharide moiety at the 4’-hydroxy position of the MbA flavonol core. Both enzymes are specific for flavonol glycosides and their respective sugar donors. This study completes the discovery of the MbA biosynthetic pathway and provides the complete set of genes to engineer MbA biosynthesis. Indeed, the authors announce their successful reconstruction of MbA biosynthesis in Nicotiana benthamiana.
Diabetes and obesity are major health challenges. Diabetes alone affects over 422 million people worldwide and is among the top ten leading causes of death. Type-2 diabetes (T2D) is characterized by the body’s inefficient use of insulin, which leads to elevated blood Glc levels with detrimental effects on different organs and increased risk of dying prematurely. The plant metabolite montbretin A (MbA) is being developed as an anti-diabetes and anti-obesity treatment due to its potent and specific inhibition of the human pancreatic a-amylase. MbA is a complex acylated flavonol glycoside formed in small amounts in the corms of montbretia (Crocosmia X crocosmiiflora), a member of the iris family, during the early summer. Unfortunately. the spatial and temporal patterns of MbA accumulation limit its supply for drug development and application. Irmisch et al. (10.1104/pp.20.00522) are exploring MbA biosynthesis with the aim of enabling the metabolic engineering of this rare and valuable compound. Genes and enzymes for the first four steps of MbA biosynthesis, starting from the flavonol precursor myricetin, have recently been identified. Now, the authors describe the gene discovery and functional characterization of the remaining two enzymes of MbA biosynthesis. The authors reveal that two UDP-glycosyltransferases, CcUGT4 and CcUGT5, catalyze consecutive reactions in the formation of the disaccharide moiety at the 4’-hydroxy position of the MbA flavonol core. Both enzymes are specific for flavonol glycosides and their respective sugar donors. This study completes the discovery of the MbA biosynthetic pathway and provides the complete set of genes to engineer MbA biosynthesis. Indeed, the authors announce their successful reconstruction of MbA biosynthesis in Nicotiana benthamiana.