Barley RIPb opens the gates for epidermal fungal penetration
Elisa Dell’Aglio
Institut National des Sciences Appliquées de Lyon
Lyon,
France
The ascomycete Blumeria graminis f. sp. hordei (Bgh) is a powdery mildew causal agent, specifically adapted to barley (Hordeum vulgare), wheat (Triticum aestivum, Triticum turgidum) and triticale (× Triticosecale). Bgh can cause up to 40% of yield losses (Draz et al., 2019) and has become a model to study the interactions between plants and leaf fungal pathogens.
On wheat leaves, Bgh forms specialized hyphae called haustoria that penetrate through epidermal cells and subsequently inject virulent factors in plant tissues, while starting nutrient uptake. As a countermeasure, the invaded cell’s morphology changes drastically: thanks to cytoskeleton reorganization, the nucleus and other organelles (e.g. mitochondria and the vacuole) relocate to the site of invasion to favor apposition of callose and other insulating molecules (Chowdhury et al., 2014). Therefore, studying the cytoskeletal dynamics is essential to explain plant defense mechanisms.
One of the most studied cytoskeleton remodelers in Arabidopsis is RACB, a RHO GTPase influencing the stability of actin filaments and microtubules (Opalski et al., 2005). During Bgh infection, a constitutively active version of the barley RACB protein favors mildew spread; conversely, downregulation of RACB leads to a lower fungal penetration rate (Schultheiss et al., 2002; Hoefle et al., 2011). However, RAC proteins are part of a complex signaling pathway. Previous studies have revealed that modulation of RACB interactors can lead to unexpected outcomes in terms of fungal penetration rates (Nottensteiner et al., 2018; Hoefle et al., 2020).
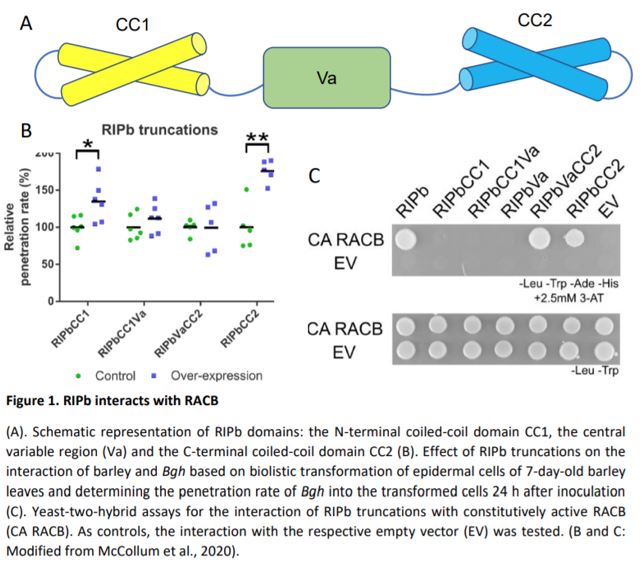 In this issue of Plant Physiology, Christopher McCollum and colleagues (McCollum et al., 2020) identify the barley (Hordeum vulgare) ROP Interactive Partner b (RIPb) as another modulator of Bgh virulence. Indeed, the authors showed that transient RIPb overexpression in barley leaves was able to increase the Bgh penetration rate by 22%.
In this issue of Plant Physiology, Christopher McCollum and colleagues (McCollum et al., 2020) identify the barley (Hordeum vulgare) ROP Interactive Partner b (RIPb) as another modulator of Bgh virulence. Indeed, the authors showed that transient RIPb overexpression in barley leaves was able to increase the Bgh penetration rate by 22%.
To understand the molecular mechanism by which RIPb promotes fungal penetration, McCollum and colleagues observed the subcellular localization of RIPb in barley epidermal cells by confocal microscopy. YFP-RIPb was present in the cytosol, at the cell periphery (likely the plasma membrane) but also in contact with microtubules. Interestingly, YFP-RIPb coexpression with a constitutively active RACB version (but not with a constitutively inactive RACB) triggered RIPb relocalization from the cytoplasm to the cell periphery. Therefore, RIPb localization appears dynamic and dependent on RACB activation status, suggesting a physical interaction between RIPb and RACB.
Yeast two-hybrid assays confirmed the physical interaction between RIPb and RACB. By testing various RIPb truncations, the authors revealed that the RIPb C-terminal coiled-coil domain (CC2) is essential for RACB interaction, while a central variable domain (VA) is important for RIPb – RIPb oligomerization. These results are in accordance with previous work that identified the conserved QWRKAA amino acid sequence, located in the CC2 domain, as the motif responsible for RIP – RAC interactions (Lavy et al., 2007). The same truncations monitored in vivo by confocal microscopy further revealed that the RIPb – RIPb interaction takes place mainly at microtubules and only in the presence of both the VA domain and of an N-terminal coiled-coil domain (CC1), while the RIPb CC2 domain is essential for the relocation of the protein at the plasma membrane in the presence of constitutively active RACB. In addition, the CC2 domain is necessary and sufficient to determine the increase in fungal penetration rate observed when RIPb is overexpressed.
To explore RACB – RIPb interaction in the context of fungal attack, RIPb and RACB-expressing leaves were inoculated with Bgh conidia. Ring-like accumulation of both proteins appeared at the sites of infection, especially around the haustorial neck of successful penetration sites but also, in some cases, at sites of repelled fungal attempts.
A phylogenetic analysis revealed that barley possesses three RIP proteins (RIPa, RIPb and RIPc), which are likely conserved in grasses (e.g. rice and Brachypodium distachyon). There is, however, very little sequence similarity between RIPs from monocots and dicots. In particular, while the C-terminal coiled-coil domain is well conserved, the N-terminal region is more divergent between the two groups. This difference suggests that dicot RIPs probably also interact with RAC proteins at the plasma membrane, but their regulation might be very different.
In conclusion, this study identified RIPb as a RACB-interacting protein that is recruited at the periphery of epidermal cells under fungal attack, via RIPb C-terminal coiled-coil domain. To better understand the role of RIPb in fungal pathogenesis, it will be important to identify additional RACB and RIPb interactions. Because of their high evolutionary divergence, it will be also important to undertake functional studies of RIPs from dicot species and assess similarities and differences in their roles and regulatory mechanisms with respect to monocots.
Literature Cited
Chowdhury J, Henderson M, Schweizer P, Burton RA, Fincher GB, Little A (2014) Differential accumulation of callose, arabinoxylan and cellulose in nonpenetrated versus penetrated papillae on leaves of barley infected with Blumeria graminis f. sp. hordei. New Phytol 204: 650-660
Draz IS, Esmail SM, Abou-Zeid MAEH, Essa TAEM (2019) Powdery mildew susceptibility of spring wheat cultivars as a major constraint on grain yield. Ann Agric Sci 64: 39-45
Hoefle C, Huesmann C, Schultheiss H, Bornke F, Hensel G, Kumlehn J, Hückelhoven R (2011) A Barley ROP GTPase ACTIVATING PROTEIN Associates with Microtubules and Regulates Entry of the Barley Powdery Mildew Fungus into Leaf Epidermal Cells. Plant Cell 23: 2422-2439
Hoefle C, McCollum C, Hückelhoven R (2020) Barley ROP-Interactive Partner-a organizes into RAC1- and MICROTUBULEASSOCIATED ROP-GTPASE ACTIVATING PROTEIN 1-dependent membrane domains. BMC Plant Biol 20: 94
Lavy M, Bloch D, Hazak O, Gutman I, Poraty L, Sorek N, Sternberg H, Yalovsky S (2007) A Novel ROP/RAC effector links cell polarity, root-meristem maintenance, and vesicle trafficking. Curr Biol 17: 947-952
Nottensteiner M, Zechmann B, McCollum C, Hückelhoven R (2018) A barley powdery mildew fungus non-autonomous retrotransposon encodes a peptide that supports penetration success on barley. J Exp Bot 69: 3745-3758
Opalski KS, Schultheiss H, Kogel KH, Hückelhoven R (2005) The receptor-like MLO protein and the RAC/ROP family G-protein RACB modulate actin reorganization in barley attacked by the biotrophic powdery mildew fungus Blumeria graminis f.sp. hordei. Plant J 41: 291-303
Schultheiss H, Dechert C, Kogel KH, Hückelhoven R (2002) A small GTP-binding host protein is required for entry of powdery mildew fungus into epidermal cells of barley. Plant Physiol 128: 1447-1454