Axis of Algae: Disruption of Basal Cell Fates in the Brown Alga Ectocarpus
Polarization may be bad for civil discourse, but sometimes polarization can be good—for example, if you’re a multicellular organism setting its body axes. In many organisms, polarity within the zygote sets the stage for an asymmetric cell division that defines the apical-basal polarity of the developing organism (reviewed in Rensing, 2016). In addition to providing insight on cellular polarity, the examination of development can provide insight into multicellularity (reviewed in Cock and Collén, 2015). Land plants establish their apical-basal axes before the first cell division– for example, in Arabidopsis thaliana, asymmetrical division of the zygote produces the root and shoot lineages. This division involves movement of the nucleus and other organelles, enlargement of the vacuole, and reorganization of microtubules.
In brown algae such as Fucus and Ectocarpus, asymmetric division of a single initial cell produces cells with apical and basal identities (see figure). The apical cell forms a branched thallus, analogous to shoots in land plants; the thallus is flattened in Fucus and filamentous in Ectocarpus. The basal cell forms filamentous rhizoids that anchor the algae to the substratum, analogous to roots in land plants. Godfroy et al. (2017) identified an Ectocarpus mutant that does not produce rhizoids (see figure) and named this mutant distag (dis), which means “detached” in Breton. Ectocarpus has alternating gametophytic and sporophytic generations, which start from a single cell and develop similar structures. The dis mutant gametophytes and sporophytes did not develop rhizoids, indicating that the rhizoids in the gametophyte and sporophyte are developmentally equivalent. The dis mutants also fail to develop rhizoids following wounding, or at secondary sites (see figure). Indeed, transcriptome analysis showed the dis mutants have similar transcriptomes to apical cells of wild type, rather than to basal cells.
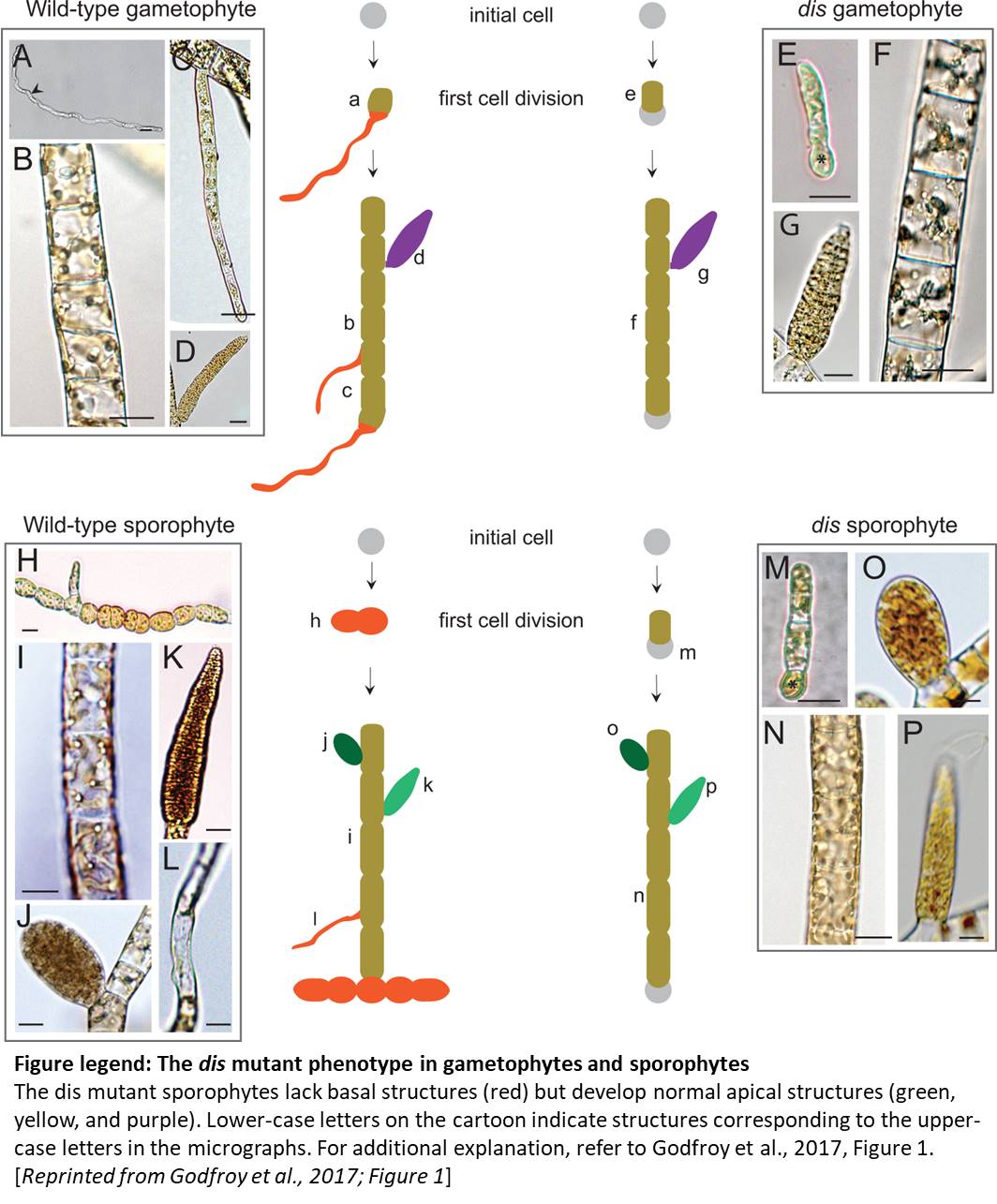 The gametophytes and sporophytes of brown algae develop from single cells outside of the parent organism (sporophyte or gametophyte), indicating that they likely establish polarity in a cell-autonomous manner. Indeed, work in Fucus implicated signaling in the apoplast and cell wall in the establishment of polarity and, indeed, transcriptome analysis of dis and apical cells showed upregulation of processes related to cell signaling, secretion and cell wall modification. Consistent with the establishment of polarity before the first cell division, the dis mutants show a strong phenotype in the initial cell, with a disordered microtubule network (more clusters of microtubules), larger cell size, altered Golgi structure (more abundant but more fragmented), and mis-positioned nucleus and centrioles. However, the plane of the first cell division was normal and, intriguingly, the observed cellular defects disappeared by 15 days after germination, indicating that DIS function is specific to the initial cell.
The gametophytes and sporophytes of brown algae develop from single cells outside of the parent organism (sporophyte or gametophyte), indicating that they likely establish polarity in a cell-autonomous manner. Indeed, work in Fucus implicated signaling in the apoplast and cell wall in the establishment of polarity and, indeed, transcriptome analysis of dis and apical cells showed upregulation of processes related to cell signaling, secretion and cell wall modification. Consistent with the establishment of polarity before the first cell division, the dis mutants show a strong phenotype in the initial cell, with a disordered microtubule network (more clusters of microtubules), larger cell size, altered Golgi structure (more abundant but more fragmented), and mis-positioned nucleus and centrioles. However, the plane of the first cell division was normal and, intriguingly, the observed cellular defects disappeared by 15 days after germination, indicating that DIS function is specific to the initial cell.
Cloning-by-sequencing, aided by the published Ectocarpus genome identified changes in one locus in both dis alleles; RNA interference targeting this locus also phenocopied the dis phenotype. This analysis showed that DIS encodes a Tubulin Binding Co-factor C (TBCC) domain protein of the TBCCd1 class. DIS expression was higher in basal cells than in apical cells. TBCC proteins have been implicated in assembly of ab-tubulin and affect cellular morphology in animals and other systems; however, although Chlamydomonas mutants defective in a related TBCCd1 protein had defects in flagella, the dis mutant gametophytes showed normal structure and function, including number of flagella, swimming, phototaxis, and fertilization, indicating that although the mutants have a disordered microtubule network, dis does not cause a global breakdown of microtubule-related processes. Moreover, pharmacological treatments with microtubule depolymerizing or stabilizing drugs (oryzalin and taxol, respectively), and with the Golgi-disrupting drug Brefeldin A failed to phenocopy the dis phenotype. Therefore, the dis mutant phenotype likely does not result from a simple problem with microtubules, but reflects a global developmental impairment of basal cell fate. The authors postulate that the mutant phenotype results because the mutants fail to “deploy” a factor that specifies cell fate—however, the exact role of the DIS TBCCd1 protein in determining or elaborating basal cell fate, and its implications for the evolution of the sporophyte-gametophyte life cycle, remain exciting possibilities for future research.
REFERENCES
Cock, J.M. and Collén, J. (2015). Independent emergence of complex multicellularity in the brown and red algae. In: Evolutionary transitions to multicellular life. Ruiz-Trillo, I. and Nedelcu, A.M. (eds) Springer, Dordrecht, pp. 335-361.
Godfroy, O., Uji, T., Nagasato, C., Lipinska, A.P., Scornet, D., Peters, A.F., Avia, K., Colin, S., Mignerot, L., Motomura, T., Cock, J.M., Coelho, S.M. (2017) DISTAG/TBCCd1 Is Required for Basal Cell Fate Determination in Ectocarpus. Plant Cell 10.1105/tpc.17.00440.
Rensing, S.A. (2016) (Why) Does Evolution Favour Embryogenesis? In Trends in Plant Science, Volume 21:562-573.