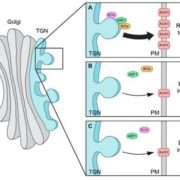
Arabidopsis FLL2 promotes liquid–liquid phase separation of polyadenylation complexes ($) (Nature)
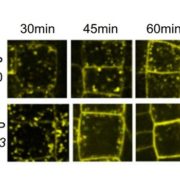
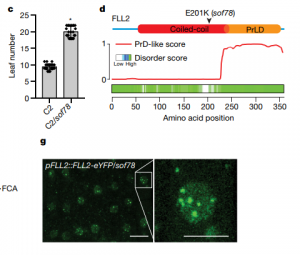 The nucleus of plants cells, like other eukaryotes, is full of non-membranous compartments separated by liquid-liquid phases. These complexes are often called nuclear bodies and concentrate proteins and nucleic acids. Disordered protein domains play a critical in their formation. Here, Fang et al. aimed to study the function of FCA, an Arabidopsis protein required for the alternative 3’-end processing of the long non-coding RNA COOLAIR that regulates the critical flowering regulator Flowering Locus C (FLC). Through a mutagenesis strategy, they searched for factors required for FCA-mediated repression of FLC. They found FLL2, a disordered protein with a coiled-coil domain. This protein colocalizes and interacts with FCA in nuclear bodies through a shared disordered prion-like domain. The mutation of FLL2 reduces the FCA nuclear body localization, and both can promote liquid-liquid phase separation in vitro and possess a liquid-like behavior in vivo. These proteins also are necessary to promote polyadenylation and are associated with the 3′ RNA-processing components. Moreover, their homologs, FLL1 and FLL3, also could carry out similar functions. This paper is a good example of how to approach to the fascinating field of disordered proteins and liquid-liquid phase separations in plants. (Summary by Facundo Romani) Nature 110.1038/s41586-019-1165-8
The nucleus of plants cells, like other eukaryotes, is full of non-membranous compartments separated by liquid-liquid phases. These complexes are often called nuclear bodies and concentrate proteins and nucleic acids. Disordered protein domains play a critical in their formation. Here, Fang et al. aimed to study the function of FCA, an Arabidopsis protein required for the alternative 3’-end processing of the long non-coding RNA COOLAIR that regulates the critical flowering regulator Flowering Locus C (FLC). Through a mutagenesis strategy, they searched for factors required for FCA-mediated repression of FLC. They found FLL2, a disordered protein with a coiled-coil domain. This protein colocalizes and interacts with FCA in nuclear bodies through a shared disordered prion-like domain. The mutation of FLL2 reduces the FCA nuclear body localization, and both can promote liquid-liquid phase separation in vitro and possess a liquid-like behavior in vivo. These proteins also are necessary to promote polyadenylation and are associated with the 3′ RNA-processing components. Moreover, their homologs, FLL1 and FLL3, also could carry out similar functions. This paper is a good example of how to approach to the fascinating field of disordered proteins and liquid-liquid phase separations in plants. (Summary by Facundo Romani) Nature 110.1038/s41586-019-1165-8