AP-2-dependent Endocytosis of BRI1
Liu et al. demonstrate how canonical tyrosine endocytic motifs control the internalization of plasma membrane proteins by the clathrin adaptor complex AP-2. The Plant Cell (2020) https://doi.org/10.1105/tpc.20.00384.
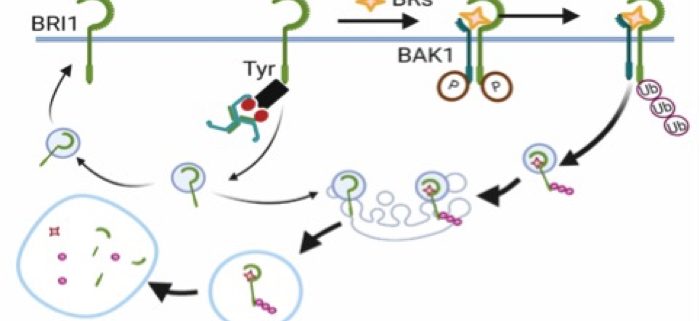
Background: In plants, plasma membrane (PM)-resident proteins are selectively engulfed into the cell, a process called endocytosis, which is crucial for signal activation or termination. Clathrin-mediated endocytosis (CME) is one of the most studied internalization routes in plants. CME and its core endocytic machinery are evolutionarily conserved across all eukaryotes. In mammals, the heterotetrameric adaptor protein complex-2 (AP-2) sorts PM cargoes for endocytosis via the recognition of motifs based on tyrosine or di-leucine in their cytoplasmic tails. However, in plants, very little is known about how PM proteins are sorted by AP-2 for CME and whether similar motifs are required.
Question: We wanted to know if AP-2 recognition motifs in PM proteins are functional in plants. We used BRASSINOSTEROID INSENSITIVE1 (BRI1), the receptor of brassinosteroid (BR) hormones, as a model system to study this.
Findings: We show that the medium subunit of AP-2, AP2M, directly binds to BRI1 in vitro and in vivo through a tyrosine-based endocytic motif present in BRI1. Mutations in this motif resulted in plants that accumulated BRI1 in the PM and were hypersensitive to BRs in Arabidopsis seedlings. Our study demonstrates that canonical tyrosine motifs are functional in plants and that they control the internalization of PM proteins by AP-2.
Next steps: We will further investigate the mechanisms of ligand-induced endocytosis of BRI1, which relies on ubiquitination.
Derui Liu et al. (2020). Endocytosis of BRASSINOSTEROID INSENSITIVE1 is Partly Driven by a Canonical Tyrosine-based Motif. Plant Cell DOI: https://doi.org/10.1105/tpc.20.00384.