Unexpected Role of a TCP Transcription Factor in Seed Oil Biosynthesis
Tianhu Sun
ORCID ID: 0000-0002-2513-1387
Plant Breeding and Genetics Section, School of Integrative Plant Science, Cornell University, Ithaca, New York 14853
Plant seed oils (such as canola oil, sunflower oil, and soybean oil) are important for the human diet and store energy vital to support seedling development. Therefore, there is tremendous interest in understanding the regulation of seed oil biosynthesis. Triacylglycerols (TAGs) are the major form of seed oil in plants, and de novo fatty acid biosynthesis in plastids provides precursors for TAG formation (Baud 2018).
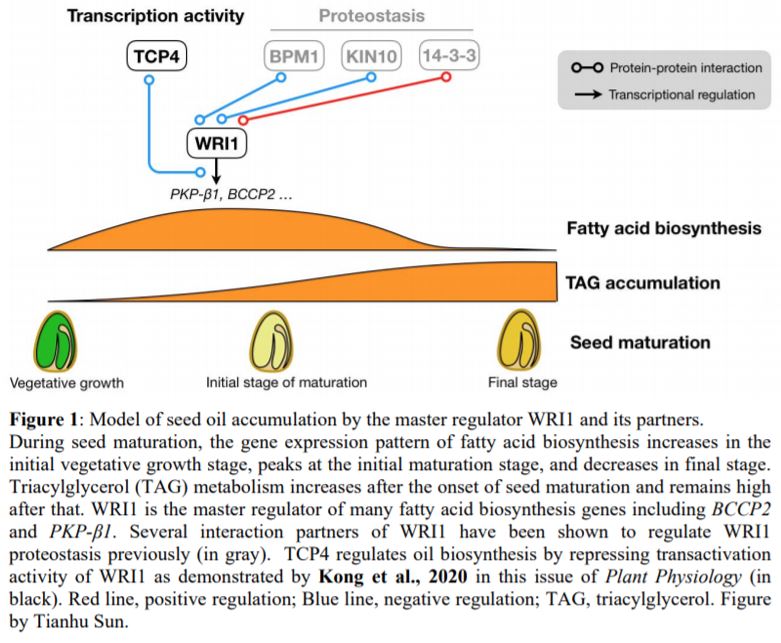 The model plant Arabidopsis thaliana (Arabidopsis), a close relative to a major oilseed crop Brassica napus, has become an ideal system to investigate seed oil biosynthesis. Over a decade ago, an Arabidopsis mutant wrinkled1 (wri1) with 80% reduction of seed oil content was isolated (Focks and Benning, 1998). It was subsequently identified as a loss-of-function mutant of WRI1, a master transcriptional regulator of fatty acid biosynthesis (Cernac and Benning, 2004). In recent years, several interaction partners of WRI1, such as BTB/POZMATH 1 (BPM1) (Chen et al., 2013), 14-3-3 proteins (Ma et al., 2016), and the protein kinase KIN10 (Zhai et al., 2018), have been identified as regulators of WRI1 proteostasis (Figure 1). However, considering the crucial role of WRI1 in seed oil accumulation, how WRI1 activity is fine-tuned to regulate fatty acid biosynthesis is still incompletely understood.
The model plant Arabidopsis thaliana (Arabidopsis), a close relative to a major oilseed crop Brassica napus, has become an ideal system to investigate seed oil biosynthesis. Over a decade ago, an Arabidopsis mutant wrinkled1 (wri1) with 80% reduction of seed oil content was isolated (Focks and Benning, 1998). It was subsequently identified as a loss-of-function mutant of WRI1, a master transcriptional regulator of fatty acid biosynthesis (Cernac and Benning, 2004). In recent years, several interaction partners of WRI1, such as BTB/POZMATH 1 (BPM1) (Chen et al., 2013), 14-3-3 proteins (Ma et al., 2016), and the protein kinase KIN10 (Zhai et al., 2018), have been identified as regulators of WRI1 proteostasis (Figure 1). However, considering the crucial role of WRI1 in seed oil accumulation, how WRI1 activity is fine-tuned to regulate fatty acid biosynthesis is still incompletely understood.
In this issue of Plant Physiology, Kong et al. (2020) provide new insight into WRI1 function by demonstrating that a TEOSINTE BRANCHED1/CYCLOIDEA/PROLIFERATING CELL FACTOR (TCP) transcription factor, TCP4, binds to WRI1 and negatively regulates seed oil biosynthesis through the repression of WRI1 transactivation activity. The authors searched for protein-protein interaction partners of WRI1 by a yeast two-hybrid screen. Several TCP transcription factors (TCP4, TCP10, and TCP24) were obtained and the interactions were further confirmed by bimolecular fluorescence complementation (BiFC). Although TCPs are involved in various plant developmental processes, their role in fatty acid biosynthesis was not documented previously.
The authors first addressed which of the identified TCPs have a role in fatty acid biosynthesis. During seed maturation, the expression of fatty acid biosynthetic genes increases during seed vegetative growth stage and peaks at the onset of seed maturation (Figure 1). The authors determined that TCP4 gene expression best fits the timing of fatty acid biosynthesis because TCP4 and TCP10 are co-expressed with WRI1, and most importantly, only TCP4 exhibited a similar temporal expression pattern to WRI1 based on the analysis of previously published gene expression datasets.
While the expression pattern of TCP4 is consistent with it having a role in fatty acid biosynthesis, the authors sought additional genetic evidence to support its participation. They examined the seed oil content of several mutant lines: single mutant tcp4, triple mutant tcp2 tcp4 tcp10, and a mutant jaw-D that shows repression of several TCP genes (including TCP2, TCP3, TCP4, TCP10, and TCP24). Compared to wild type, all lines with decreased expression of TCP4 showed increased seed oil levels while the mutation or repression of other genes showed minor additive effect. These results clearly indicate that TCP4 is the major repressor of seed oil content.
Next, the authors asked how TCP4 works together with WRI1 to regulate oil biosynthesis. A convenient and robust method to functionally validate oil biosynthesis regulators is to transiently express the regulator in Nicotiana benthamiana leaves and then examine oil accumulation (Ma et al., 2015). Kong et al. (2020) tested the effects of different combinations of TCPs and WRI1 with this method. As expected, expression of WRI1 induced oil accumulation. Co-expression of TCP4 and WRI1 led to a decreased level of oil production as compared to TCP4 alone, while co-expression TCP10 or TCP24 with WRI1 showed no change comparing to WRI1 alone. These results clearly show that TCP4 represses WRI1-induced oil biosynthesis.
Previous work showed that fatty acid biosynthesis genes BCCP2 and PKP-β1 are direct transcriptional targets of WRI1 (Schommer et al., 2008). In this work, the authors found that these WRI1 target genes are down-regulated in a TCP4 over-accumulation line. Next, they examined the effect of TCP4 and WRI1 interaction on WRI1 function. Although TCP4 itself does not show transactivation activity, the addition of TCP4 to WRI1 represses the transactivation activity of WRI1 on the promoters of BCCP2 and PKP-β1. However, whether TCP4 competes at the WRI1 binding site, or inhibits WRI1 activity though binding to a novel site of these promoters needs to be further addressed.
In this study, Kong et al. (2020) uncovered the unexpected role of the TCP4 transcription factor in seed oil biosynthesis through its interaction with the master regulator WRI1, thus shedding light on the complicated regulatory network of seed oil biosynthesis. WRI1 plays central role in seed oil production and several post-translational regulators have been identified to maintain the homeostasis of WRI1 (Figure 1). This work provides further evidence of the requirement of brakes as well as accelerators in seed oil biosynthesis.
This work also raises additional questions. For example, the molecular mechanism of how TCP4 represses WRI1 transactivation is not yet clear and the authors proposed several possible models which could be tested in future. Not only oil, but also other storage compounds such as starch and protein will accumulate during the seed maturation process. Thus, the partitioning of carbon between these compounds is assumed to be balanced at certain stages. However, a recent study showed that the seed oil yield can be boosted without compromising storage protein and starch in seeds, and also without negatively impacting shoot biomass (Li et al., 2020). Therefore, the negative control of WRI1 and fatty acid production by TCP4 may serve to fine-tune fatty acid biosynthesis in an as yet undefined tempo-spatial manner.
LITERATURE CITED
Baud S (2018) Seeds as oil factories. Plant Reproduction 31:213-235
Cernac A, Benning C (2004) WRINKLED1 encodes an AP2/EREB domain protein involved in the control of storage compound biosynthesis in Arabidopsis. Plant J 40: 575-585
Chen L, Lee JH, Weber H, Tohge T, Witt S, Roje S, Fernie AR, Hellmann H (2013) Arabidopsis BPM Proteins Function as Substrate Adaptors to aCULLIN3 Based E3 Ligase to Affect Fatty Acid Metabolism in Plants. Plant Cell 25:2253-2264
Focks N, Benning C (1998) wrinkled1: A novel, low-seed-oil mutant of Arabidopsis with a deficiency in the seed-specific regulation of carbohydrate metabolism. Plant Physiol 118: 91-101
Li N, Meng H, Li S, Zhang Z, Zhao X, Wang S, Liu A, Li Q, Song Q, Li X, Guo L (2020) Two Plastid Fatty Acid Exporters Contribute to Seed Oil Accumulation in Arabidopsis. Plant Physiol 182:1910-1919
Ma W, Kong Q, Mantyla JJ, Yang Y, Ohlrogge JB, Benning C (2016) 14-3-3 protein mediates plant seed oil biosynthesis through interaction with AtWRI1. Plant J 88: 228-235
Ma W, Kong Q, Grix M, Mantyla JJ, Yang Y, Benning C, Ohlrogge JB (2015) Deletion of a C-terminal intrinsically disordered region of WRINKLED1 affects its stability and enhances oil accumulation in Arabidopsis. Plant J 83: 864-874
Schommer C, Palatnik JF, Aggarwal P, Chetelat A, Cubas P, Farmer EE, Nath U, Weigel D (2008) Control of jasmonate biosynthesis and senescence by miR319 targets. PLoS Biol 6: e230
Zhai Z, Keereetaweep J, Liu H, Feil R, Lunn JE, Shanklin J (2018) Trehalose 6-Phosphate Positively Regulates Fatty Acid Synthesis by Stabilizing WRINKLED1. Plant Cell 30: 2616-2627