A spatiotemporal molecular switch governs plant asymmetric cell division (Nature Plants)
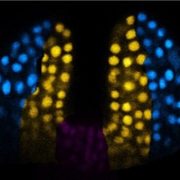
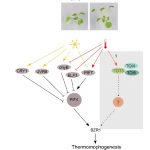
 In multicellular organisms, stem cells produce various cell types through asymmetric cell division (ACD), which is achieved is through polarization of cell-fate determinant proteins in the ACD progenitor cells. In Arabidopsis stomatal development, an ACD progenitor cell, the meristemoid mother cell (MMC), produces daughter cells with different cell-fates: a meristemoid and a stomatal lineage ground cell (SLGC). MMCs emerge from protodermal cells (PrC) through the activity of the SPEECHLESS (SPCH) transcription factor, which promotes expression of the scaffolding protein BREAKING OF ASYMMETRY IN THE STOMATAL LINEAGE (BASL). BASL polarizes at the cell membrane and establishes cellular asymmetry together with other scaffolding proteins to assemble the polarity complex. Differences in the composition of the polarity complex in MMCs vs SLGCs trigger either cell-division or fate specification/differentiation, respectively, however the mechanism hasn’t been delineated. A recent paper by Guo and colleagues identified the phosphatase BSL1 as part of the polarity complex through its interaction with BASL. The authors show that BSL1 polarizes to the cell membrane in a BASL-dependent manner at the onset of mitosis and promotes repartitioning of the BIN2 kinase to the nucleus through its phosphatase activity. Parallel to this, BSL1 activates the YODA MAPK signaling pathway which leads to SPCH degradation and inhibition of cell division in the SLGC. This work demonstrates that BSL1 polarization at the entry of MMC cell division acts as a spatiotemporal molecular switch that triggers essential changes in the composition of the polarity complex and thus fine-tunes MMC ACD. (Summary by Jesus Leon @jesussaur) Nature Plants 10.1038/s41477-021-00906-0
In multicellular organisms, stem cells produce various cell types through asymmetric cell division (ACD), which is achieved is through polarization of cell-fate determinant proteins in the ACD progenitor cells. In Arabidopsis stomatal development, an ACD progenitor cell, the meristemoid mother cell (MMC), produces daughter cells with different cell-fates: a meristemoid and a stomatal lineage ground cell (SLGC). MMCs emerge from protodermal cells (PrC) through the activity of the SPEECHLESS (SPCH) transcription factor, which promotes expression of the scaffolding protein BREAKING OF ASYMMETRY IN THE STOMATAL LINEAGE (BASL). BASL polarizes at the cell membrane and establishes cellular asymmetry together with other scaffolding proteins to assemble the polarity complex. Differences in the composition of the polarity complex in MMCs vs SLGCs trigger either cell-division or fate specification/differentiation, respectively, however the mechanism hasn’t been delineated. A recent paper by Guo and colleagues identified the phosphatase BSL1 as part of the polarity complex through its interaction with BASL. The authors show that BSL1 polarizes to the cell membrane in a BASL-dependent manner at the onset of mitosis and promotes repartitioning of the BIN2 kinase to the nucleus through its phosphatase activity. Parallel to this, BSL1 activates the YODA MAPK signaling pathway which leads to SPCH degradation and inhibition of cell division in the SLGC. This work demonstrates that BSL1 polarization at the entry of MMC cell division acts as a spatiotemporal molecular switch that triggers essential changes in the composition of the polarity complex and thus fine-tunes MMC ACD. (Summary by Jesus Leon @jesussaur) Nature Plants 10.1038/s41477-021-00906-0