A SNARE Protein Involved in H+-ATPase Trafficking
Membrane proteins are synthesized in the endoplasmic reticulum and are trafficked through the Golgi, from which they are sorted to the plasma membrane, tonoplast, or other target membranes. In all eukaryotes, secretory traffic to the plasma membrane is mediated by SNARE proteins. In Arabidopsis, three Qa-SNAREs, commonly referred to as syntaxins, namely SYP121 (Syntaxin of Plants121, or SYR1/PEN1) and its close homologs SYP122 and SYP132, are ubiquitously expressed at the plasma membrane throughout plant development. Although SYP121 and SYP122 drive the bulk of secretory 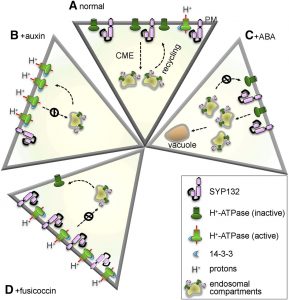 traffic to the plasma membrane, they do not seem to involved in trafficking of one of the most import plasma membrane proteins, the H+-ATPase. Plasma membrane H+-ATPases extrude protons against an electrochemical gradient with the expenditure of ATP to generate strong proton motive forces that energize H+-coupled transport and ion flux at the plasma membrane. These plasma membrane H+-ATPases drive various processes including osmotic nutrient uptake and stomatal opening, they facilitate salt tolerance and regulate intracellular pH, and they are essential for apoplast acidification and plastic cell wall loosening during vegetative growth. Although SYP132 expression makes up only a minor portion of the Qa-SNAREs at the plasma membrane, Xia et al. (10.1104/pp.19.00266) reveal that SYP132 plays an important role in regulating the density of the H+-ATPases in the plasma membrane. Interestingly, SYP132 expression is tightly regulated by auxin and increased SYP132 expression reduces the amount of H+-ATPase proteins at the plasma membrane. The physiological consequences of SYP132 overexpression include reduced apoplast acidification and suppressed vegetative growth. Thus, SYP132 plays unexpected and vital roles in auxin-regulated H+-ATPase traffic and associated functions at the plasma membrane.
traffic to the plasma membrane, they do not seem to involved in trafficking of one of the most import plasma membrane proteins, the H+-ATPase. Plasma membrane H+-ATPases extrude protons against an electrochemical gradient with the expenditure of ATP to generate strong proton motive forces that energize H+-coupled transport and ion flux at the plasma membrane. These plasma membrane H+-ATPases drive various processes including osmotic nutrient uptake and stomatal opening, they facilitate salt tolerance and regulate intracellular pH, and they are essential for apoplast acidification and plastic cell wall loosening during vegetative growth. Although SYP132 expression makes up only a minor portion of the Qa-SNAREs at the plasma membrane, Xia et al. (10.1104/pp.19.00266) reveal that SYP132 plays an important role in regulating the density of the H+-ATPases in the plasma membrane. Interestingly, SYP132 expression is tightly regulated by auxin and increased SYP132 expression reduces the amount of H+-ATPase proteins at the plasma membrane. The physiological consequences of SYP132 overexpression include reduced apoplast acidification and suppressed vegetative growth. Thus, SYP132 plays unexpected and vital roles in auxin-regulated H+-ATPase traffic and associated functions at the plasma membrane.