A single gene underlies the dynamic evolution of poplar sex determination (Nature Plants)
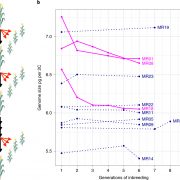
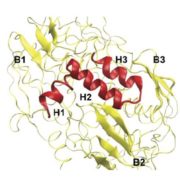
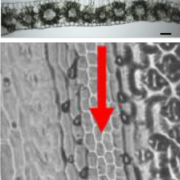
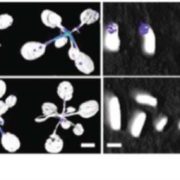
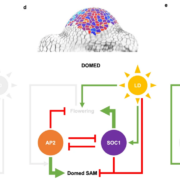
 Dioecy (male and female flowers residing on distinct individuals) has independently arisen several times in angiosperms, yet the genetic basis of dioecy remains obscure. Here, Müller et al. reveal a single gene that acts as a sex-determination switch throughout the Populus genus. A negative regulator of cytokinin, the Populus ortholog of ARABIDOPSIS RESPONSE REGULATOR 17 (ARR17) initiates female development when switched ‘on’, and male development when ‘off’. In the Y chromosome, partial ARR17 duplicates lie adjacent to known sex-linked genes. The duplicates are inversely repeated, suggesting that upon transcription double-stranded RNA may form and be processed into small RNAs that can trigger RNA-directed DNA methylation, resulting in male-specific silencing of ARR17. CRISPR– Cas9 mutants confirmed the feminizing role of ARR17, with female arr17 plants lacking carpels but producing functional stamens, while male arr17 lines were phenotypically unchanged. To investigate whether the ARR17 system is conserved, white poplar, which is an unusual Populus in that it possesses ZW sex chromosomes instead of XY, was sequenced. Remarkably, a natural arr17 knockout was discovered on the Z chromosome, meaning males lack ARR17 (ZZ-male, ZW-female), which likely facilitated the evolution of white poplar’s ZW-system. Examining the evolution of Populus sex-determining regions revealed two independent origins of ARR17 repeats, with one seemingly predating Populus speciation. Future discoveries in other lineages will enable exciting comparisons with this elegant single-gene switch, unveiling connections between sex determination and species evolution. (Summary by Caroline Dowling @CarolineD0wling) Nature Plants 10.1038/s41477-020-0672-9
Dioecy (male and female flowers residing on distinct individuals) has independently arisen several times in angiosperms, yet the genetic basis of dioecy remains obscure. Here, Müller et al. reveal a single gene that acts as a sex-determination switch throughout the Populus genus. A negative regulator of cytokinin, the Populus ortholog of ARABIDOPSIS RESPONSE REGULATOR 17 (ARR17) initiates female development when switched ‘on’, and male development when ‘off’. In the Y chromosome, partial ARR17 duplicates lie adjacent to known sex-linked genes. The duplicates are inversely repeated, suggesting that upon transcription double-stranded RNA may form and be processed into small RNAs that can trigger RNA-directed DNA methylation, resulting in male-specific silencing of ARR17. CRISPR– Cas9 mutants confirmed the feminizing role of ARR17, with female arr17 plants lacking carpels but producing functional stamens, while male arr17 lines were phenotypically unchanged. To investigate whether the ARR17 system is conserved, white poplar, which is an unusual Populus in that it possesses ZW sex chromosomes instead of XY, was sequenced. Remarkably, a natural arr17 knockout was discovered on the Z chromosome, meaning males lack ARR17 (ZZ-male, ZW-female), which likely facilitated the evolution of white poplar’s ZW-system. Examining the evolution of Populus sex-determining regions revealed two independent origins of ARR17 repeats, with one seemingly predating Populus speciation. Future discoveries in other lineages will enable exciting comparisons with this elegant single-gene switch, unveiling connections between sex determination and species evolution. (Summary by Caroline Dowling @CarolineD0wling) Nature Plants 10.1038/s41477-020-0672-9