A sensor kinase controls turgor-driven plant infection by the rice blast fungus ($) (Nature)
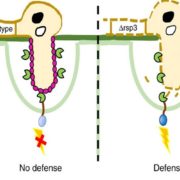
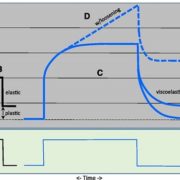
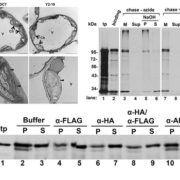
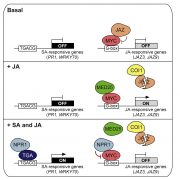
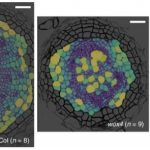
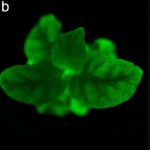
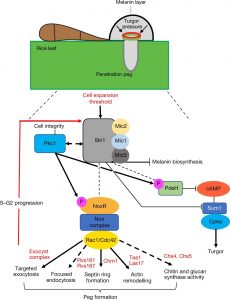 Magnaporthe oryzae, the causal organism of blast disease in rice and wheat, is the most devasting pathogen in rice production. During infection, it develops a germ tube that forms an infection structure called the appressorium. Through septin-mediated reorganization of the cytoskeleton, a high amount of turgor pressure is generated that allows it to rupture the leaf cuticle. Ryder et al. used a combination of cell biology, molecular genetics and mathematical modelling to decipher the molecular mechanisms regulating appressorium-generated turgor pressure during plant infection. Typically, accumulation of glycerol and a melanin-rich cell wall is necessary for turgor generation. Treatment with glycerol or a melanin biosynthesis inhibitor interferes with turgor accumulation and septin reorganization, thus, demonstrating that a critical threshold of cellular turgor is required for septin organization at the appressorium pore and that this event is necessary for plant infection. The authors identified a kinase, Sln1, that functions as a turgor sensor and localizes at the appressorium pore in a pressure-dependent way. Sln1 also negatively regulates melanin biosynthesis and glycerol production. The Sln1 knock-out strain Δsln1 displays a non-functional appressorium with disrupted septin organization, excess intracellular appressorium turgor and a hyper-melanized appressorium phenotype. Taken together, Ryder et al demonstrated that turgor-mediated infection by the rice blast pathogen is mediated by a turgor sensor kinase, Sln1. (Summary by Toluwase Olukayode) Nature 10.1038/s41586-019-1637-x
Magnaporthe oryzae, the causal organism of blast disease in rice and wheat, is the most devasting pathogen in rice production. During infection, it develops a germ tube that forms an infection structure called the appressorium. Through septin-mediated reorganization of the cytoskeleton, a high amount of turgor pressure is generated that allows it to rupture the leaf cuticle. Ryder et al. used a combination of cell biology, molecular genetics and mathematical modelling to decipher the molecular mechanisms regulating appressorium-generated turgor pressure during plant infection. Typically, accumulation of glycerol and a melanin-rich cell wall is necessary for turgor generation. Treatment with glycerol or a melanin biosynthesis inhibitor interferes with turgor accumulation and septin reorganization, thus, demonstrating that a critical threshold of cellular turgor is required for septin organization at the appressorium pore and that this event is necessary for plant infection. The authors identified a kinase, Sln1, that functions as a turgor sensor and localizes at the appressorium pore in a pressure-dependent way. Sln1 also negatively regulates melanin biosynthesis and glycerol production. The Sln1 knock-out strain Δsln1 displays a non-functional appressorium with disrupted septin organization, excess intracellular appressorium turgor and a hyper-melanized appressorium phenotype. Taken together, Ryder et al demonstrated that turgor-mediated infection by the rice blast pathogen is mediated by a turgor sensor kinase, Sln1. (Summary by Toluwase Olukayode) Nature 10.1038/s41586-019-1637-x