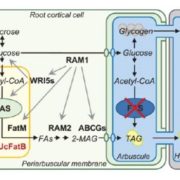
A SAC phosphoinositide phosphatase controls rice development via hydrolyzing PI4P and PI(4,5)P2 (Plant Physiol)
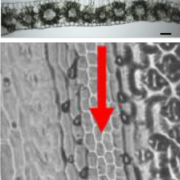
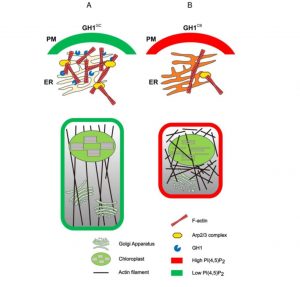 As their name suggests, phosphoinositide (PI) phosphatases remove phosphates from phosphoinositides (try saying that fast!). Because the phosphorylation status of a membrane-bound PI determines which proteins it interacts with, PI phosphatases and kinases contribute to membrane functions and dynamics. Here, Guo et al. explore the function of a SAC family PI phosphatase, which they identified through a mapping study as GRAIN NUMBER AND PLANT HEIGHT1 (GH1). In the rice variety CB, there is a premature stop codon in the gene that renders it nonfunctional, leading to an overaccumulation of PI4P and PI(4,5)P2, as well as abnormalities in the actin cytoskeleton and vesicle trafficking. In vitro studies suggest that the Arp2/3 complex, which functions as a nucleator of actin filament dynamics, might be the target of the elevated levels of PIs. (Summary by Mary Williams) Plant Physiol. 10.1104/pp.19.01131
As their name suggests, phosphoinositide (PI) phosphatases remove phosphates from phosphoinositides (try saying that fast!). Because the phosphorylation status of a membrane-bound PI determines which proteins it interacts with, PI phosphatases and kinases contribute to membrane functions and dynamics. Here, Guo et al. explore the function of a SAC family PI phosphatase, which they identified through a mapping study as GRAIN NUMBER AND PLANT HEIGHT1 (GH1). In the rice variety CB, there is a premature stop codon in the gene that renders it nonfunctional, leading to an overaccumulation of PI4P and PI(4,5)P2, as well as abnormalities in the actin cytoskeleton and vesicle trafficking. In vitro studies suggest that the Arp2/3 complex, which functions as a nucleator of actin filament dynamics, might be the target of the elevated levels of PIs. (Summary by Mary Williams) Plant Physiol. 10.1104/pp.19.01131