A prion-like domain in ELF3 functions as a thermosensor in Arabidopsis (Nature)
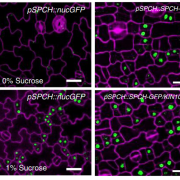
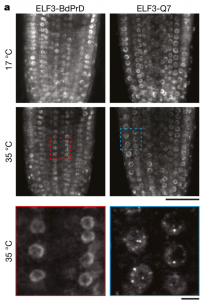 As sessile organisms, sensing the external conditions is critical for plants to complete their life cycle and temperature is one of the major factors. In Arabidopsis, the evening complex senses the temperature and it consists of EARLY FLOWERING3 (ELF3), a scaffolding protein; ELF4, helical protein; LUX ARRYTHMO (LUX), a DNA binding protein. However, the molecular mechanism behind the temperature sensing by evening complex remains elusive. In this paper, Jung et al. reported the phase transition of ELF3 from active to inactive state as a molecular mechanism for sensing the temperature. ELF3 has polyglutamine (polyQ) repeats whose length correlates weakly with the temperature sensitivity and are present within a predicted prion domain (PrD). PrD length varies between plants growing in warmer temperatures compared to the cold temperatures. ELF3-GFP protein forms a diffused signal and speckles at lower and higher temperatures respectively in planta and in heterologous expression system (yeast). This phase differentiation is reversible as the speckles disappear when cells are transferred from the higher to lower temperature and correlates with the evening complex function in sensing the temperature over a short period of time. Thus, this paper advances our understanding of ELF3 in sensing the temperature reversibly by undergoing a phase transition and has the potential implication especially, as global temperature continues to increase. (Summary by Vijaya Batthula @Vijaya_Batthula). Nature. 10.1038/s41586-020-2644-7
As sessile organisms, sensing the external conditions is critical for plants to complete their life cycle and temperature is one of the major factors. In Arabidopsis, the evening complex senses the temperature and it consists of EARLY FLOWERING3 (ELF3), a scaffolding protein; ELF4, helical protein; LUX ARRYTHMO (LUX), a DNA binding protein. However, the molecular mechanism behind the temperature sensing by evening complex remains elusive. In this paper, Jung et al. reported the phase transition of ELF3 from active to inactive state as a molecular mechanism for sensing the temperature. ELF3 has polyglutamine (polyQ) repeats whose length correlates weakly with the temperature sensitivity and are present within a predicted prion domain (PrD). PrD length varies between plants growing in warmer temperatures compared to the cold temperatures. ELF3-GFP protein forms a diffused signal and speckles at lower and higher temperatures respectively in planta and in heterologous expression system (yeast). This phase differentiation is reversible as the speckles disappear when cells are transferred from the higher to lower temperature and correlates with the evening complex function in sensing the temperature over a short period of time. Thus, this paper advances our understanding of ELF3 in sensing the temperature reversibly by undergoing a phase transition and has the potential implication especially, as global temperature continues to increase. (Summary by Vijaya Batthula @Vijaya_Batthula). Nature. 10.1038/s41586-020-2644-7