A practical guide for fluorophore selection for FRET experiments in plants (Plant Direct)
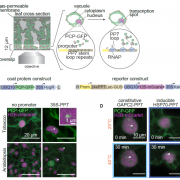
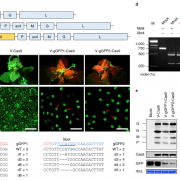
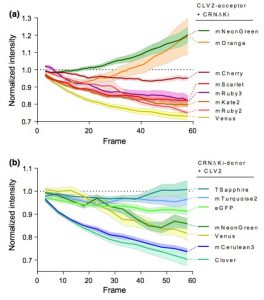 Protein-protein interactions modulate the activities of many proteins, i.e., the signaling specificity of Receptor-like kinases (RLK). Förster Resonance Energy Transfer (FRET) allows the study of the dynamics of protein-protein interactions in live plants; this technique is based on energy transfer from a fluorescent donor to an acceptor fluorophore. There are many fluorophore pairs available for FRET experiments, but most of them have been characterized in vitro or in mammalian cell systems. In this study, Denay and collaborators tested the performance of 12 fluorophore pairs in a plant cell system. They focused on membrane proteins; for this, they chose the CORYNE-CLAVATA2 (CRN-CLV2) dimer. Protein localization analysis allowed the study of the fluorophore effect on protein folding and function since a correct CRN/CLV2 interaction is necessary for a correct export from the endoplasmic reticulum to the plasma membrane. Other analyses on the fluorophores included sensitivity to photobleaching and noise-to-signal ratio in the cellular context. Measurement of FRET efficiency confirmed eGFP-mCherry as a good FRET pair, as well as other combinations with similar performance, i.e., Venus-mKate2, Venus-mRuby3, mCerulean3-mNeonGreen, and mTurquoise2-Venus. Furthermore, the authors identified combinations of three different fluorophores that would allow the study of interactions between three proteins, protein complexes, or the measurement of competitive interactions. (Summary by Humberto Herrera-Ubaldo) Plant Direct. 10.1002/pld3.189
Protein-protein interactions modulate the activities of many proteins, i.e., the signaling specificity of Receptor-like kinases (RLK). Förster Resonance Energy Transfer (FRET) allows the study of the dynamics of protein-protein interactions in live plants; this technique is based on energy transfer from a fluorescent donor to an acceptor fluorophore. There are many fluorophore pairs available for FRET experiments, but most of them have been characterized in vitro or in mammalian cell systems. In this study, Denay and collaborators tested the performance of 12 fluorophore pairs in a plant cell system. They focused on membrane proteins; for this, they chose the CORYNE-CLAVATA2 (CRN-CLV2) dimer. Protein localization analysis allowed the study of the fluorophore effect on protein folding and function since a correct CRN/CLV2 interaction is necessary for a correct export from the endoplasmic reticulum to the plasma membrane. Other analyses on the fluorophores included sensitivity to photobleaching and noise-to-signal ratio in the cellular context. Measurement of FRET efficiency confirmed eGFP-mCherry as a good FRET pair, as well as other combinations with similar performance, i.e., Venus-mKate2, Venus-mRuby3, mCerulean3-mNeonGreen, and mTurquoise2-Venus. Furthermore, the authors identified combinations of three different fluorophores that would allow the study of interactions between three proteins, protein complexes, or the measurement of competitive interactions. (Summary by Humberto Herrera-Ubaldo) Plant Direct. 10.1002/pld3.189