A new node in mediating crosstalk between the proteasomal and autophagic degradation pathways (Plant Cell)
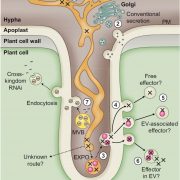
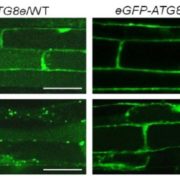
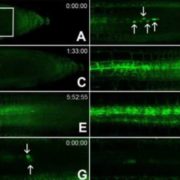
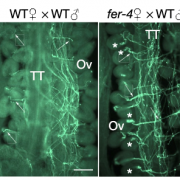
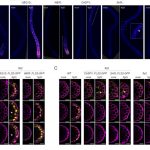
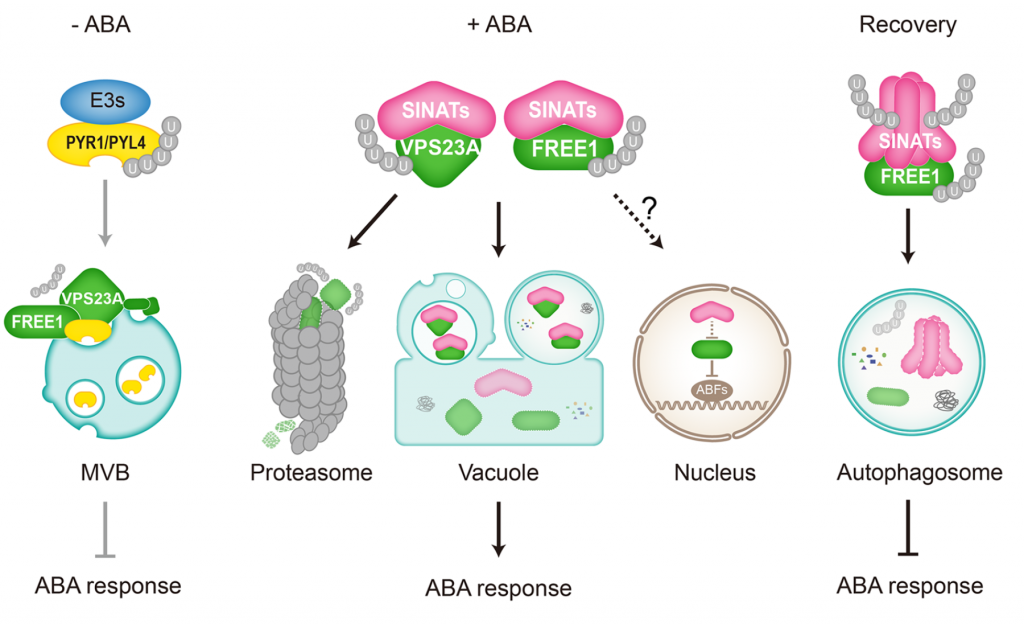 Protein degradation is mediated by several systems: the ubiquitin-proteasome system, multi-vesicle body-mediated vacuolar sorting (MVB), and the autophagy-vacuole pathway. However, the communication between these different protein degradation systems are less characterized. Here, Xia et al. report a new node in mediating crosstalk between the proteasomal and autophagic degradation pathways. They show that the SEVEN IN ABSENTIA of ARABIDOPSIS THALIANA (SINATs), which are RING-type E3 ubiquitin ligases, modulate the protein stability of two proteins involved in mediating autophagy, FYVE DOMAIN PROTEIN (FREE1) and VACUOLAR PROTEIN SORTING 23A (VPS23A). SINAT1/2/4 are localized to the endomembrane and autophagosome vesicles and interact with FREE1 and VPS23A. SINATs control the ubiquitination and co-degradation with FREE1 and VPS23A through the MVB and autophagy-vacuole degradation pathway. Mutants of vps23a and freel knockdown are hypersensitive to ABA treatment, while overexpression of SINATs show increased sensitivity to ABA. Interestingly, during post-ABA recovery, oligomers of SINATs are co-degraded with FREE1 through the autophagy pathway. Overall, this study provides new insights into the interplay between different protein degradation pathways and ABA signalling. (Summary by Min May Wong @wongminmay) Plant Cell 10.1105/tpc.20.00267
Protein degradation is mediated by several systems: the ubiquitin-proteasome system, multi-vesicle body-mediated vacuolar sorting (MVB), and the autophagy-vacuole pathway. However, the communication between these different protein degradation systems are less characterized. Here, Xia et al. report a new node in mediating crosstalk between the proteasomal and autophagic degradation pathways. They show that the SEVEN IN ABSENTIA of ARABIDOPSIS THALIANA (SINATs), which are RING-type E3 ubiquitin ligases, modulate the protein stability of two proteins involved in mediating autophagy, FYVE DOMAIN PROTEIN (FREE1) and VACUOLAR PROTEIN SORTING 23A (VPS23A). SINAT1/2/4 are localized to the endomembrane and autophagosome vesicles and interact with FREE1 and VPS23A. SINATs control the ubiquitination and co-degradation with FREE1 and VPS23A through the MVB and autophagy-vacuole degradation pathway. Mutants of vps23a and freel knockdown are hypersensitive to ABA treatment, while overexpression of SINATs show increased sensitivity to ABA. Interestingly, during post-ABA recovery, oligomers of SINATs are co-degraded with FREE1 through the autophagy pathway. Overall, this study provides new insights into the interplay between different protein degradation pathways and ABA signalling. (Summary by Min May Wong @wongminmay) Plant Cell 10.1105/tpc.20.00267