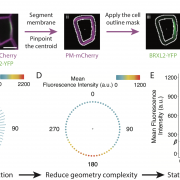
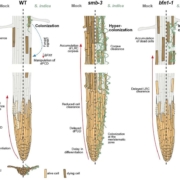
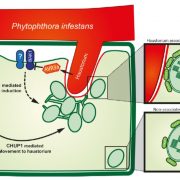
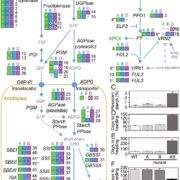
A Kinesin-14 motor activates neocentromeres to promote meiotic drive in maize
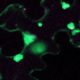
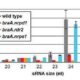
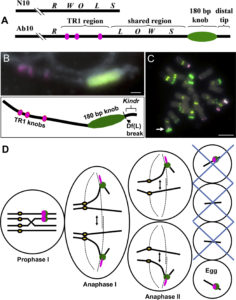 Meiotic drive is essentially a subversion of meiosis such that particular regions or alleles are preferentially favored for transmission to the progeny. Abnormal chromosome 10 (Ab10) is a classic example of meiotic drive in maize that converts heterochromatic chromosomal knobs into motile ‘neocentromeres’. When Ab10 is present in the genome, all knobs move earlier and faster than centromeres, ultimately delivering knobbed chromatids into upper and lower cells of the tetrads during meiosis. This ensures preferential segregation of Ab10 and knobs to the progeny, as the lower cell goes on to form the egg. Dawe et al. used genomic approaches to identify a previously unknown region in Ab10 containing at least eight tandemly arrayed copies of Kinesin driver (Kindr). Two Ab10 mutants that lack meiotic drive turned out to be epimutants of kindr. These epimutants have increased DNA methylation directly over the promoter and small RNA accumulation on the promoter and first intron, resulting in decreases in Kindr gene expression. RNAi suppression of kindr showed the expected reduction in preferential transmission, proving its requirement in neocentromeric activity and meiotic drive. The authors show that KINDR belongs to the Kinesin-14A family, and at least in vitro, generates faster microtubule gliding capacity than its closest maize homolog ZmKIN11. Kindr and ZMKIN11 share ~94% amino acid identity, and phylogenetic analysis suggests a divergence time of ~11.8 MY. This period coincides with the divergence of maize and sorghum. Sorghum diverged from maize ~12 MYAa anddoes not have maize-like knobs, whereas Tripsacum dactyloides, which diverged from maize ~1 MYA, has knobs similar to that of maize. KINDR specifically binds to knobs with 180 bp repeats and not those containing TR1 repeats. Ab10 has a profound effect on maize, adding ~500 Mb of DNA to the genome and affecting segregation of a substantial number of genes. This potential of centromeric sequences to drive their preferential transmission may have led to epigenetic mechanisms to counter their inheritance. (Summary by Sunil Kumar Kenchanmane Raju) Cell 10.1016/j.cell.2018.03.009
Meiotic drive is essentially a subversion of meiosis such that particular regions or alleles are preferentially favored for transmission to the progeny. Abnormal chromosome 10 (Ab10) is a classic example of meiotic drive in maize that converts heterochromatic chromosomal knobs into motile ‘neocentromeres’. When Ab10 is present in the genome, all knobs move earlier and faster than centromeres, ultimately delivering knobbed chromatids into upper and lower cells of the tetrads during meiosis. This ensures preferential segregation of Ab10 and knobs to the progeny, as the lower cell goes on to form the egg. Dawe et al. used genomic approaches to identify a previously unknown region in Ab10 containing at least eight tandemly arrayed copies of Kinesin driver (Kindr). Two Ab10 mutants that lack meiotic drive turned out to be epimutants of kindr. These epimutants have increased DNA methylation directly over the promoter and small RNA accumulation on the promoter and first intron, resulting in decreases in Kindr gene expression. RNAi suppression of kindr showed the expected reduction in preferential transmission, proving its requirement in neocentromeric activity and meiotic drive. The authors show that KINDR belongs to the Kinesin-14A family, and at least in vitro, generates faster microtubule gliding capacity than its closest maize homolog ZmKIN11. Kindr and ZMKIN11 share ~94% amino acid identity, and phylogenetic analysis suggests a divergence time of ~11.8 MY. This period coincides with the divergence of maize and sorghum. Sorghum diverged from maize ~12 MYAa anddoes not have maize-like knobs, whereas Tripsacum dactyloides, which diverged from maize ~1 MYA, has knobs similar to that of maize. KINDR specifically binds to knobs with 180 bp repeats and not those containing TR1 repeats. Ab10 has a profound effect on maize, adding ~500 Mb of DNA to the genome and affecting segregation of a substantial number of genes. This potential of centromeric sequences to drive their preferential transmission may have led to epigenetic mechanisms to counter their inheritance. (Summary by Sunil Kumar Kenchanmane Raju) Cell 10.1016/j.cell.2018.03.009