A Closer Look at the Fatty Acid Assembly Line
Nam et al. describe a general method to analyze and quantify the fatty acid biosynthetic profile of any sample with the use of an aspartate protease and LC-MS/MS. Plant Cell https://doi.org/10.1105/tpc.19.00954
By Lauren M. Jenkinsa,b, Jeong-Won Nama, and Doug K. Allena,b (aDonald Danforth Plant Science Center and bUSDA-ARS)
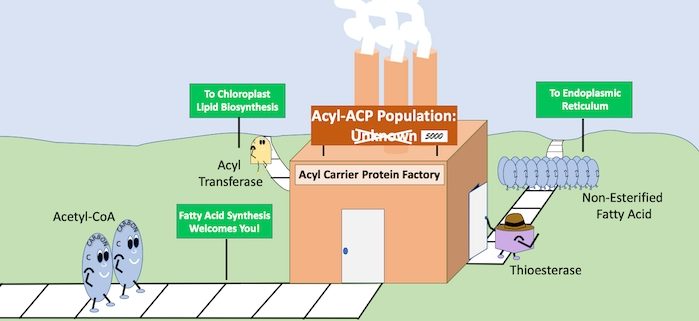
Background: Lipids are a major and most energy-dense storage reserve in nature; therefore, understanding how lipids are made has important applications to human nutrition and biofuel production. The energy in lipids is a result of fatty acids that are synthesized by adding two carbons at a time to an elongating acyl-chain. In plants, bacteria, and the mitochondria of eukaryotes, fatty acid de novo synthesis occurs with the elongating chain attached to a scaffold protein referred to as the acyl carrier protein (ACP). The process of fatty acid de novo synthesis is a repeated set of reactions involving condensation, reduction, dehydration and a second reduction of the acyl chain while attached to the ACP. Though the steps are known, questions remain about the regulation and dynamic operation of the cycle, leaving a void in our understanding.
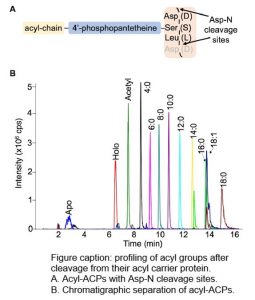 Question: What are the levels of acyl-ACPs in different plant tissues? Given that acyl groups attach to the ACP through a highly conserved amino acid sequence, can we use this to develop a thorough method to quantify individual acyl-ACPs using a more contemporary approach of mass spectrometry?
Question: What are the levels of acyl-ACPs in different plant tissues? Given that acyl groups attach to the ACP through a highly conserved amino acid sequence, can we use this to develop a thorough method to quantify individual acyl-ACPs using a more contemporary approach of mass spectrometry?
Findings: We synthesized 15N isotopically labeled acyl-ACPs to quantify the acyl-ACP pools in seed and leaf metabolism. The highly conserved amino acid sequence that connects the acyl chain to the ACP scaffold can be conveniently cleaved with an aspartate protease before analysis by liquid chromatography-mass spectrometry. We quantified expected acyl-ACPs (2-18 carbons in length) from Camelina sativa and additionally observed intermediates of the acyl-ACP synthetic process (3-hydroxyacyl-ACPs and 2,3-trans-enoyl-ACPs) owing to the sensitivity of the method. Interestingly, we also observed unanticipated medium-chain monounsaturated (10 and 14 carbons) and long-chain polyunsaturated (16 carbons, 3 double bonds) acyl-ACPs suggesting that our understanding of lipid metabolism is incomplete.
Next steps: This method provides the tool to fill important gaps in our understanding of fatty acid de novo synthesis processes by assessing differences among species, tissue types, and environmental conditions. With the help of stable isotopes, we can use this tool to trace changes in fatty acid synthetic rates and quantitatively describe poorly understood lipid remodeling events.
Jeong-Won Nam, Lauren M. Jenkins, Jia Li, Bradley S. Evans, Jan G. Jaworski, and Doug K. Allen (2020). A General Method for Quantification and Discovery of Acyl Groups Attached to Acyl Carrier Proteins in Fatty Acid Metabolism using LC-MS/MS. Plant Cell. DOI: https://doi.org/10.1105/tpc.19.00954