Vacuolar H+-ATPase Regulates Al Resistance
In acidic soils, phytotoxic levels of active aluminum (Al) are released from Al-containing minerals when the pH drops to 5 or lower. These active Al 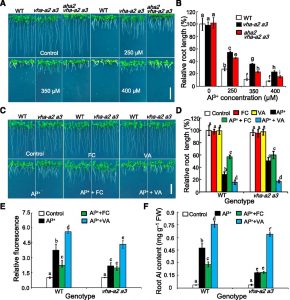 forms, primarily Al3+, often threaten root vitality and inhibit primary root elongation, thus limiting the acquisition of nutrient elements and water necessary for crop growth. Thus, Al toxicity is a major constraint for crop production in acidic soils. Plants cope with aluminum (Al) toxicity by secreting organic acids (OAs) into the apoplastic space where they chelate Al3+ or by storing OAs in the vacuole where they also act to bind Al3+. Zhang et al. (10.1104/pp.19.00626) provide insights into these two mechanisms of Al resistance in Arabidopsis. Interestingly, in response to Al3+ exposure, Arabidopsis plants showed decreased vacuolar H+ pump activity and reduced expression of VHA-a2 and VHA-a3, which were accompanied by increased plasma membrane H+ pump (PM H+-ATPase) activity. Mutation of the vacuolar H+-translocating adenosine triphosphatase (H+-ATPase) subunit a2 (VHA-a2) and VHA-a3 of the vacuolar H+-ATPase enhances Al resistance in Arabidopsis. vha-a2 vha-a3 mutant plants displayed less Al sensitivity with less Al accumulation in roots compared to wild-type plants when grown under excessive Al3+. Genetic analysis of plants with altered PM H+-ATPase activity established a correlation between Al-induced increase in PM H+-ATPase activity and enhanced Al resistance in vha-a2 vha-a3 plants. External OAs, such as malate and citrate, whose secretion is driven by PM H+-ATPase, increased with PM H+-ATPase activity upon Al stress. On the other hand, elevated secretion of malate and citrate in vha-a2 vha-a3 root exudates was likely related to impaired vacuolar sequestration. These results suggest that coordination of vacuolar H+-ATPase and PM H+-ATPase dictates the distribution of OAs into either the vacuolar lumen or the apoplastic space that, in turn, determines Al tolerance capacity in plants.
forms, primarily Al3+, often threaten root vitality and inhibit primary root elongation, thus limiting the acquisition of nutrient elements and water necessary for crop growth. Thus, Al toxicity is a major constraint for crop production in acidic soils. Plants cope with aluminum (Al) toxicity by secreting organic acids (OAs) into the apoplastic space where they chelate Al3+ or by storing OAs in the vacuole where they also act to bind Al3+. Zhang et al. (10.1104/pp.19.00626) provide insights into these two mechanisms of Al resistance in Arabidopsis. Interestingly, in response to Al3+ exposure, Arabidopsis plants showed decreased vacuolar H+ pump activity and reduced expression of VHA-a2 and VHA-a3, which were accompanied by increased plasma membrane H+ pump (PM H+-ATPase) activity. Mutation of the vacuolar H+-translocating adenosine triphosphatase (H+-ATPase) subunit a2 (VHA-a2) and VHA-a3 of the vacuolar H+-ATPase enhances Al resistance in Arabidopsis. vha-a2 vha-a3 mutant plants displayed less Al sensitivity with less Al accumulation in roots compared to wild-type plants when grown under excessive Al3+. Genetic analysis of plants with altered PM H+-ATPase activity established a correlation between Al-induced increase in PM H+-ATPase activity and enhanced Al resistance in vha-a2 vha-a3 plants. External OAs, such as malate and citrate, whose secretion is driven by PM H+-ATPase, increased with PM H+-ATPase activity upon Al stress. On the other hand, elevated secretion of malate and citrate in vha-a2 vha-a3 root exudates was likely related to impaired vacuolar sequestration. These results suggest that coordination of vacuolar H+-ATPase and PM H+-ATPase dictates the distribution of OAs into either the vacuolar lumen or the apoplastic space that, in turn, determines Al tolerance capacity in plants.