Using cryo-EM to solve the structures of proteins involved in starch degradation
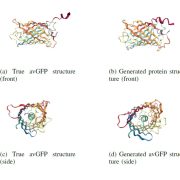
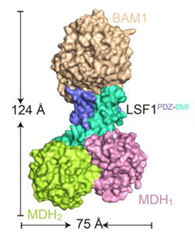 Starch is synthesized in the chloroplasts of leaves during the day and degraded at night. BAM1 (β-AMYLASE 1) catalyzes starch degradation and interacts with the non-catalytic glucan phosphatase called LSF1 (LIKE SEX FOUR 1) and the plastid localised MDH (MALATE DEHYDROGENASE). However, we don’t fully understand how these proteins function together. Here, Liu et al. determined the BAM1-LSF1-MDH structure to 3 Å resolution using cryo-EM. The structure is a dumbbell shape with BAM1 at one end, a MDH dimer at the other end and LSF1 connecting the two. Unfortunately, the DSP (dual specificity domain) and CBM (carbohydrate-binding module) domains of LSF1 were not detected in the structure. Therefore in vitro cross linking coupled with mass spectrometry was used to show that the LSF1 CBM domain was close to the BAM1 catalytic domain. Thus, the LSF1 CBM domain most likely provides polyglucan substrates for BAM1 mediated degradation. The structure also showed that MDH interacts with LSF1 via a latch and gate mechanism that is dependent on three aspartates in MDH. When these Asps are mutated, LSF1 becomes less stable, showing that MDH enhances LSF1 stability. Hence solving the BAM1-LSF1-MDH structure has provided greater insight into the roles of LSF1 and MDH in starch degradation. (Summary by Rose McNelly @Rose_McN) Plant Cell 10.1093/plcell/koad259
Starch is synthesized in the chloroplasts of leaves during the day and degraded at night. BAM1 (β-AMYLASE 1) catalyzes starch degradation and interacts with the non-catalytic glucan phosphatase called LSF1 (LIKE SEX FOUR 1) and the plastid localised MDH (MALATE DEHYDROGENASE). However, we don’t fully understand how these proteins function together. Here, Liu et al. determined the BAM1-LSF1-MDH structure to 3 Å resolution using cryo-EM. The structure is a dumbbell shape with BAM1 at one end, a MDH dimer at the other end and LSF1 connecting the two. Unfortunately, the DSP (dual specificity domain) and CBM (carbohydrate-binding module) domains of LSF1 were not detected in the structure. Therefore in vitro cross linking coupled with mass spectrometry was used to show that the LSF1 CBM domain was close to the BAM1 catalytic domain. Thus, the LSF1 CBM domain most likely provides polyglucan substrates for BAM1 mediated degradation. The structure also showed that MDH interacts with LSF1 via a latch and gate mechanism that is dependent on three aspartates in MDH. When these Asps are mutated, LSF1 becomes less stable, showing that MDH enhances LSF1 stability. Hence solving the BAM1-LSF1-MDH structure has provided greater insight into the roles of LSF1 and MDH in starch degradation. (Summary by Rose McNelly @Rose_McN) Plant Cell 10.1093/plcell/koad259