Two pathways for trafficking the borate receptor BOR1
Polar localization of plasma membrane proteins is an important way cells regulate signaling pathways, transport across membranes, and growth. Polarization has obvious cellular effects, but localization of membrane components also contributes significantly to processes such as tip growth in root hairs and pollen tubes, phytohormone signaling, and directional micronutrient transport (Grunewald and Friml, 2010; Leitner et al., 2010; Luschnig and Vert, 2014; Šamaj et al., 2006). For example, in transport of the essential micronutrient boron, the borate exporter BOR1 localizes to the membranes of pericycle cells, where it helps to load boron into the xylem (Takano et al., 2002). BOR1 levels in the plasma membrane change in response to boron levels, with high levels of boron inducing internalization and degradation of BOR1 in the vacuole (Kasai et al., 2014).
In their recent report, Yoshinari et al. explore the separate endocytic pathways regulating BOR1 localization to the plasma membrane and degradation in the vacuole. In the trafficking of BOR1, recycling between the plasma membrane and trans-Golgi network/early endosomes (TGN/EE) maintains the polar localization of BOR1, and a degradation pathway from the plasma membrane to multivesicular bodies/late endosomes (MVB/LE) helps BOR1 make its way to the vacuole. The main question asked by Yoshinari et al. is what pathways mediate the polar localization and vacuolar sorting of BOR1? As shown in this report, the two pathways that manage BOR1 membrane localization include one that relies on the clathrin adaptor protein 2 (AP2) for BOR1 polarization at the plasma membrane, and a clathrin/AP2-independent pathway that functions in BOR1 degradation.
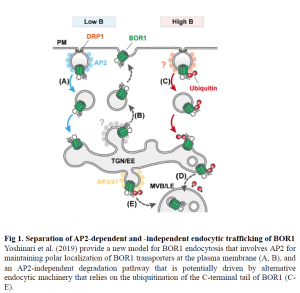 As observed for the PIN auxin transport proteins, endocytic recycling is a crucial part of maintaining BOR1 polarization (Kleine-Vehn et al., 2011). Several plasma membrane proteins share a YXXF motif recognized by AP subunits that sort or target them for vesicle transport (Park et al., 2013; Teh et al., 2013; Wang et al., 2016). In plants, loss-of-function mutants of AP2 subunits have pleotropic effects at the cellular and physiological levels, most likely because of the misregulation of the plasma membrane proteins that they help transport/localize (Fan et al., 2013; Yamaoka et al., 2013). To separate the endocytic pathways involved in BOR1 polar localization and degradation, Yoshinari et al. used BOR1-GFP constructs expressed in ap2m mutants and observed a disrupted localization pattern and a reduced level of internalization from the plasma membrane compared to wild-type. However, BOR1 endocytosis initiated by high boron conditions was not impaired in ap2m mutants, suggesting that the degradation pathways of BOR1 is AP2-independent. Indeed, high boron-induced internalization of BOR1 was only affected by a K590A point mutation, which affects ubiquitination of the C-terminal tail of BOR1. It is interesting to note that the C-terminal tail seems to be involved in both endocytic polarization and degradation pathways, but not exclusively through AP2 or other clathrin-mediated endocytic machinery. This dual regulation at the C-terminus provides new evidence of how endocytic regulator proteins potentially recognize multiple interaction sites within their target membrane proteins.
As observed for the PIN auxin transport proteins, endocytic recycling is a crucial part of maintaining BOR1 polarization (Kleine-Vehn et al., 2011). Several plasma membrane proteins share a YXXF motif recognized by AP subunits that sort or target them for vesicle transport (Park et al., 2013; Teh et al., 2013; Wang et al., 2016). In plants, loss-of-function mutants of AP2 subunits have pleotropic effects at the cellular and physiological levels, most likely because of the misregulation of the plasma membrane proteins that they help transport/localize (Fan et al., 2013; Yamaoka et al., 2013). To separate the endocytic pathways involved in BOR1 polar localization and degradation, Yoshinari et al. used BOR1-GFP constructs expressed in ap2m mutants and observed a disrupted localization pattern and a reduced level of internalization from the plasma membrane compared to wild-type. However, BOR1 endocytosis initiated by high boron conditions was not impaired in ap2m mutants, suggesting that the degradation pathways of BOR1 is AP2-independent. Indeed, high boron-induced internalization of BOR1 was only affected by a K590A point mutation, which affects ubiquitination of the C-terminal tail of BOR1. It is interesting to note that the C-terminal tail seems to be involved in both endocytic polarization and degradation pathways, but not exclusively through AP2 or other clathrin-mediated endocytic machinery. This dual regulation at the C-terminus provides new evidence of how endocytic regulator proteins potentially recognize multiple interaction sites within their target membrane proteins.
The BOR1 and PIN proteins include a YXXF motif, but interestingly, Yoshinari et al. show that altering this motif does not affect BOR1-AP2 interactions; rather, these interactions occur through domains residing on the C-terminal tail, suggesting that BOR1 internalization occurs through its interaction with AP2 is unique from other AP subunits and does not require the conserved YXXF motif. It will be interesting to follow the influence this result has on future characterization of how BOR1 protein structure contributes to the dynamics of its interactions with AP proteins and how these multiple interaction domains help specialize BOR1 localization. Moreover, this work points to clathrin- or AP-independent endocytic pathways at the plasma membrane that may help specialize the regulation of transport and signaling molecules during plant growth and environmental responses (Fig 1). It also identifies where more questions could be asked to improve our descriptions of endocytic specialization and protein recycling vs. degradation, and how these independent pathways function together to coordinate cell function.
References
Fan, L., Hao, H., Xue, Y., Zhang, L., Song, K., Ding, Z., Botella, M.A., Wang, H., and Lin, J. (2013). Dynamic analysis of Arabidopsis AP2 σ subunit reveals a key role in clathrin-mediated endocytosis and plant development. Dev. Camb. Engl. 140, 3826–3837.
Grunewald, W., and Friml, J. (2010). The march of the PINs: developmental plasticity by dynamic polar targeting in plant cells. EMBO J. 29, 2700–2714.
Kasai, K., Takano, J., and Fujiwara, T. (2014). Analysis of endocytosis and ubiquitination of the BOR1 transporter. Methods Mol. Biol. Clifton NJ 1209, 203–217.
Kleine-Vehn, J., Wabnik, K., Martinière, A., Łangowski, Ł., Willig, K., Naramoto, S., Leitner, J., Tanaka, H., Jakobs, S., Robert, S., et al. (2011). Recycling, clustering, and endocytosis jointly maintain PIN auxin carrier polarity at the plasma membrane. Mol. Syst. Biol. 7, 540.
Leitner, D., Klepsch, S., Ptashnyk, M., Marchant, A., Kirk, G.J.D., Schnepf, A., and Roose, T. (2010). A dynamic model of nutrient uptake by root hairs. New Phytol. 185, 792–802.
Luschnig, C., and Vert, G. (2014). The dynamics of plant plasma membrane proteins: PINs and beyond. Development 141, 2924–2938.
Park, M., Song, K., Reichardt, I., Kim, H., Mayer, U., Stierhof, Y.-D., Hwang, I., and Jürgens, G. (2013). Arabidopsis μ-adaptin subunit AP1M of adaptor protein complex 1 mediates late secretory and vacuolar traffic and is required for growth. Proc. Natl. Acad. Sci. U. S. A. 110, 10318–10323.
Šamaj, J., Müller, J., Beck, M., Böhm, N., and Menzel, D. (2006). Vesicular trafficking, cytoskeleton and signalling in root hairs and pollen tubes. Trends Plant Sci. 11, 594–600.
Takano, J., Noguchi, K., Yasumori, M., Kobayashi, M., Gajdos, Z., Miwa, K., Hayashi, H., Yoneyama, T., and Fujiwara, T. (2002). Arabidopsis boron transporter for xylem loading. Nature 420, 337–340.
Teh, O.-K., Shimono, Y., Shirakawa, M., Fukao, Y., Tamura, K., Shimada, T., and Hara-Nishimura, I. (2013). The AP-1 μ adaptin is required for KNOLLE localization at the cell plate to mediate cytokinesis in Arabidopsis. Plant Cell Physiol. 54, 838–847.
Wang, C., Hu, T., Yan, X., Meng, T., Wang, Y., Wang, Q., Zhang, X., Gu, Y., Sánchez-Rodríguez, C., Gadeyne, A., et al. (2016). Differential Regulation of Clathrin and Its Adaptor Proteins during Membrane Recruitment for Endocytosis. Plant Physiol. 171, 215–229.
Yamaoka, S., Shimono, Y., Shirakawa, M., Fukao, Y., Kawase, T., Hatsugai, N., Tamura, K., Shimada, T., and Hara-Nishimura, I. (2013). Identification and dynamics of Arabidopsis adaptor protein-2 complex and its involvement in floral organ development. Plant Cell 25, 2958–2969.
Yoshinari A., Hosokawa T., Amano T., Beier M.P., Kunieda T., Shimada T., Hara-Nishimura I., Naito S., Takano J. (2019). Polar Localization of the Borate Exporter BOR1 Requires AP2-Dependent Endocytosis. Plant Physiol. 179, xxx-xxx. (https://doi.org/10.1104/pp.19.00180)



