Transport-coupled ubiquitination of BOR1
Yoshinari et al. demonstrate that boron-dependent poly-ubiquitination and subsequent degradation of BOR1 is coupled with its boron transport activity. Plant Cell https://bit.ly/2JYEooF
By Akira Yoshinari
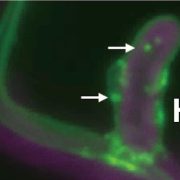
Background: Abundance and activity of nutrient transporters are continuously tuned to both maintain nutrient availability and avoid over-accumulation of nutrients to a toxic level. Boron (B) is an essential element for plants and is transported in plant tissues by the boric acid/borate transporter BOR1 under low-B conditions. BOR1 is localized in the plasma membrane under low-B conditions, but is rapidly internalized and degraded when plants are exposed to sufficient concentrations of B.
Question: Boric acid induces ubiquitination of BOR1 that initiates internalization of BOR1 and subsequent transport to the vacuole. We wanted to elucidate the mechanism for boric acid sensing that promotes ubiquitination of BOR1.
Findings: We demonstrated that BOR1 undergoes K63-linked poly-ubiquitination. We performed a forward genetic screen using BOR1-GFP to identify genes that promote ubiquitination and degradation of BOR1. Through this screen, we isolated seven independent mutants whose BOR1-GFP was stable under high-B conditions. Unexpectedly, all of the mutants carried mis-sense mutations within the BOR1-GFP transgene used as a marker. Interestingly, four out of seven mutants had mutations in the vicinity of the substrate-binding pocket of BOR1, which prompted us to test relationships between B-transport activity and ubiquitination of BOR1. Our results showed that the B-transport activity of BOR1 is necessary for its poly-ubiquitination, suggesting that BOR1 ubiquitination requires the conformation change accompanying the B transport. Our findings suggest that BOR1 is a transporter-receptor—a so-called “transceptor”—that promotes own ubiquitination and degradation according to local B concentrations.
Next steps: Future research will address how the conformational change of BOR1 during B transport influences BOR1 ubiquitination. Especially, the structural roles of amino acid residues essential for BOR1 degradation but not for B-transport activity need to be elucidated.
Akira Yoshinari, Takuya Hosokawa, Marcel Pascal Beier, Keishi Oshima, Yuka Ogino, Chiaki Hori, Taichi E Takasuka, Yoichiro Fukao, Toru Fujiwara, Junpei Takano (2021). Transport-coupled ubiquitination of the borate transporter BOR1 for its boron-dependent degradation. Plant Cell. https://bit.ly/2JYEooF