The E3 ubiquitin ligases PUB25 and PUB26 dynamically regulate cold stress responses in plants
Wang et al. discovered how plants dynamically respond to cold stress mediated by the E3 ligases PUB25 and PUB26.
https://doi.org/10.1093/plcell/koad159
By Xi Wang1, Xiaoyan Zhang1, Chunpeng Song3, Zhizhong Gong1,2, Shuhua Yang1 and Yanglin Ding1
1State Key Laboratory of Plant Environmental Resilience, College of Biological Sciences, China Agricultural University, Beijing 100193, China
2School of Life Sciences, Institute of Life Science and Green Development, Hebei University, Baoding, 071002, China.
3Institute of Plant Stress Biology, Collaborative Innovation Center of Crop Stress Biology, Henan University, Kaifeng, China,
Background: Plant cold stress responses inhibit growth by repressing cell division and expansion. Strict regulation of cold responsive genes balances growth and cold stress responses. For example, the expression of C-REPEAT BINDING FACTOR/DEHYDRATION-RESPONSIVE ELEMENT BINDING PROTEIN1 (CBF/DREB1) genes increases rapidly at the early stages of cold stress; however, their expression is inhibited at the late stages of cold stress. Ubiquitination is a conserved post-translational modification that modulates protein turnover and activity depending on the number and location of attached ubiquitin moieties. However, it remains unclear whether ubiquitination regulates the timing and degree of cold stress responses in plants by dynamically modulating cold responsive gene expression.
Question: Do the E3 ubiquitin ligases PLANT U-BOX 25 (PUB25) and PUB26 affect cold-regulated gene expression during cold stress in plants? Do PUB25 and PUB26 attach different ubiquitin chains to transcriptional factor INDUCER OF CBF EXPRESSION1 (ICE1) during cold stress? What is the detailed mechanism?
Findings: We discovered that PUB25 and PUB26 attach both K48- and K63-linked ubiquitin chains to ICE1 during cold stress. Notably, PUB25 and PUB26 attached a K63-linked ubiquitin chain to ICE1 after one hour of cold treatment; this modification may be required to stabilize ICE1, which then induces CBF expression. By contrast, K48-linked ICE1 ubiquitination increased after six hours of cold stress; this modification may attenuate the initial cold response. In addition, PUB25 and PUB26 mediated K48- and K63-linked ubiquitination of the transcription factor MYB15 during cold treatment. Furthermore, ICE1 interacted with MYB15 and repressed its binding to the promoters of CBFs. This study thus unravels a mechanism by which PUB25 and PUB26 add different polyubiquitin chains to ICE1 and MYB15 to modulate their stability, thereby regulating the timing and degree of cold stress responses in plants.
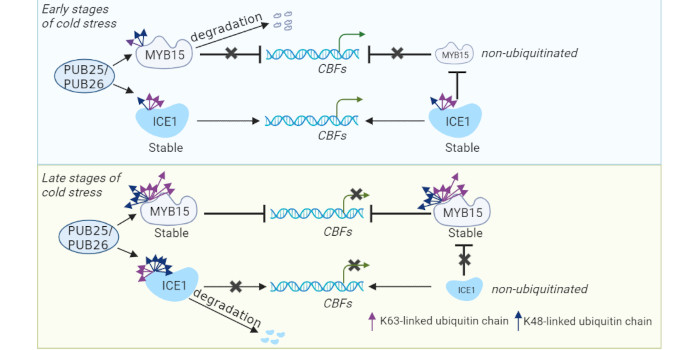
Next steps: How PUB25/PUB26-mediated ubiquitination crosstalks with phosphorylation at the different stages of cold stress is an important question. How many proteins are attached different ubiquitin chains in regulating cold stress in a time-dependent manner is another key issue to be investigated in future studies.
Reference:
Xi Wang, Xiaoyan Zhang, Chunpeng Song, Zhizhong Gong, Shuhua Yang, Yanglin Ding (2023) PUB25 and PUB26 dynamically modulate ICE1 stability via differential ubiquitination during cold stress in Arabidopsis. https://doi.org/10.1093/plcell/koad159