Timing is everything: MND1 regulates meristem phase change in barley
Lena Maria Müller
Boyce Thompson Institute, Ithaca, NY 14853, USA
0000-0002-6036-4514
The shoot and inflorescence architecture of grasses are characterized by complex branching patterns, which contribute to the great diversity of this plant clade (Whipple 2017). During crop domestication, plants were selected for a variety of body plans (Pautler et al., 2013). For example, in contrast to its highly branched ancestor teosinte, today’s maize was selected to form very few secondary shoots (tillers). This body plan allows for high planting density. Conversely, breeding of other cereal crops such as rice or barley (Hordeum vulgare) focused on yield increase by breeding for lines with increased tiller number, which consequently resulted in higher numbers of inflorescences and grains.
All vegetative and reproductive aboveground tissue is generated by the pluripotent stem cells of the shoot apical meristem (SAM). In grasses, the vegetative SAM controls shoot architecture by producing intercalary meristems, which control the elongation of internodes and thus regulate shoot growth, and by generating leaf primordia and leaf axillary meristems, which give rise to tillers and thus influence shoot branching (Pautler et al., 2013). Once vegetative development is complete, the plant transitions into the reproductive phase. This so-called floral transition is orchestrated by the vegetative SAM, which undergoes a meristem phase change and is converted into the reproductive inflorescence meristem, which then controls inflorescence development (Tanaka et al., 2013). Because vegetative and reproductive development are regulated by different phases of the same meristematic tissue, there is often a trade-off between the two. To optimize the ratio between vegetative and reproductive biomass (e.g., leaf and inflorescence number) in cereal crops, it is important to understand how different meristem activities are coordinated across the shoot.
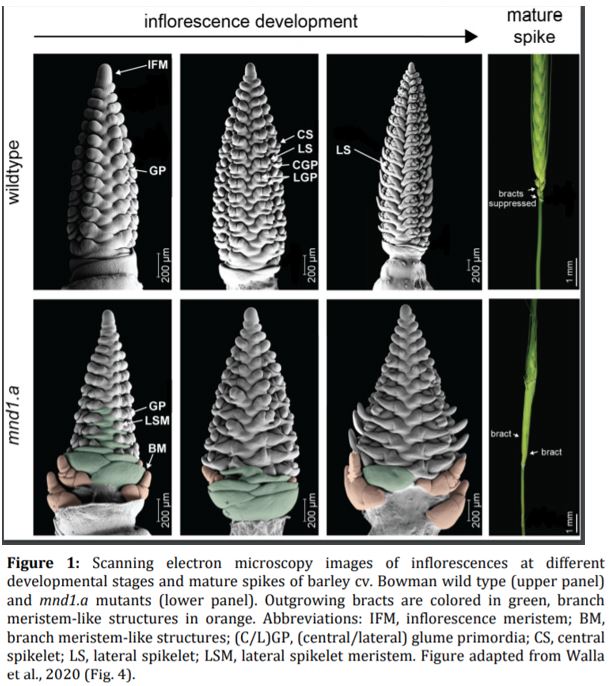 In this issue of Plant Physiology, Walla et al. report the identification of MANY NODED DWARF1 (MND1) as an important regulator of barley meristem development and phase change. Careful macro- and microscopic phenotyping revealed that mnd1.a mutants display a prolonged vegetative growth phase and a delayed transition to the reproductive phase. While wild-type plants stopped producing vegetative biomass (leaves and tillers) after 5 weeks and flowered shortly thereafter, the vegetative SAM of mnd1.a mutants remained indeterminate. This indeterminacy resulted in the continuous production of leaf primordia and axillary meristems in the mnd1.a mutant, which consequently resulted in higher leaf and tiller numbers, and increased the lifespan of mnd1.a plants to more than 12 months (as opposed to around 3 months in the wild type).
In this issue of Plant Physiology, Walla et al. report the identification of MANY NODED DWARF1 (MND1) as an important regulator of barley meristem development and phase change. Careful macro- and microscopic phenotyping revealed that mnd1.a mutants display a prolonged vegetative growth phase and a delayed transition to the reproductive phase. While wild-type plants stopped producing vegetative biomass (leaves and tillers) after 5 weeks and flowered shortly thereafter, the vegetative SAM of mnd1.a mutants remained indeterminate. This indeterminacy resulted in the continuous production of leaf primordia and axillary meristems in the mnd1.a mutant, which consequently resulted in higher leaf and tiller numbers, and increased the lifespan of mnd1.a plants to more than 12 months (as opposed to around 3 months in the wild type).
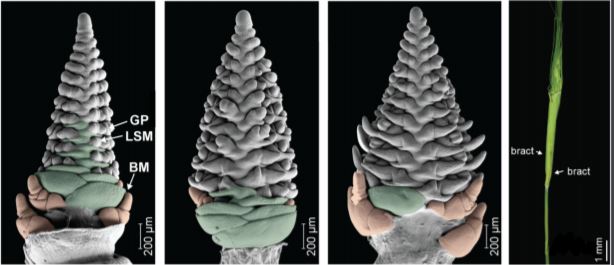
Walla et al. found that the mnd1.a mutation not only affected the shoot architecture, but also led to impaired reproductive development and the production of fewer grains. Barley inflorescence (spike) development is coordinated by the inflorescence meristem in a process roughly analogous to vegetative branch development (Tanaka et al., 2013). In wild-type plants, the inflorescence meristem produces a number of secondary meristems, which include bract (leaf) meristems, whose outgrowth is mostly arrested, and spikelet meristems, which are formed in the bract axils and give rise to the floral organs. Using scanning electron microscopy, Walla et al. found that the mnd1.a mutant was severely impaired in inflorescence development (Figure 1). Most strikingly, mnd1.a inflorescences displayed bract de-repression resulting in the formation of leaf-like organs enclosing the spike, and formed branch meristem-like structures at their base. Branch meristems are usually not present in the un-branched barley inflorescence but common in other grass species with highly branched inflorescences, such as rice (Oryza sativa) (McKim et al., 2018). Walla et al. determined that these unusual branch meristem-like structures resulted from a reversion of reproductive spikelet meristems at the base of the inflorescence. This further supports the hypothesis that mnd1.a mutants are defective in coordinated meristem transitions, which leads to pleiotropic phenotypes including an overproduction of vegetative biomass and decreased reproductive biomass, and consequently impacts plant lifespan and seed production.
The mnd1.a mutant originated spontaneously in the barley cultivar “Mesa” and subsequently was introgressed into the “Bowman” cultivar by backcrossing, resulting in a near isogenic Bowman line carrying the Mesa mnd1.a allele. Making use of sequence variation in the inflorescence transcriptomes of this mnd1.a line in comparison to the Bowman wild type, Walla et al. were able to identify a small insertion in an acyl-CoA N-acyltransferase gene, which resulted in a premature stop codon, as the cause of the mnd1.a mutant phenotype. Consistent with a presumed function in meristem phase transition, RNA in situ hybridization revealed that MND1 is specifically transcribed in axillary meristems close to the vegetative SAM, as well as in SAMs undergoing vegetative-to-reproductive phase transition.
Acyl-CoA N-acyltransferases are enzymes involved in lysine acetylation of various substrates. While Walla et al. did not observe aberrant patterns of lysine acetylation in inflorescences of mnd1.a mutants (possibly due to the highly restricted expression domain of MND1), related proteins in other plants have been shown to be involved in epigenetic regulation of gene expression by modifying histones (Song et al., 2015; Liao et al., 2016). To determine if barley MND1 also influences gene expression, Walla et al. compared the transcriptome of mnd1.a and wild-type inflorescence tissues at different developmental stages. The authors identified a number of known genes involved in floral development as well as cell cycle regulators to be differentially expressed in mnt1.a relative to the wild type. Most notably, the microRNAs miR156 and miR173, which were described as sequentially expressed master-regulators of meristem phase change in other plant species (Wu et al., 2009; Wang et al., 2015), were mis-expressed in mnd1.a meristems: While in wild-type SAMs miR156 levels decreased over time during vegetative development, they remained higher in mnd1.a. Conversely, in wild-type SAMs, miR172 levels increased over time, and this increase was delayed in mnd1.a. This lag of miR156 down-regulation and miR172 up-regulation during barley development —possibly directly caused by a disruption of MND1-mediated epigenetic regulation of their expression—in SAMs of mnd1.a mutants could very well explain the observed defects in meristem phase transitions.
While further research is needed to prove MND1 indeed acts as an epigenetic regulator of meristem phase transition, the research presented by Walla et al. nicely shows that knock-out of a single gene can have broad consequences for plant vegetative and reproductive development. Precisely tweaking the timing of meristem phase transitions might be one avenue to breed cereal crops with a desired shoot architecture and seed yield.
References
Liao C-J, Lai Z, Lee S, Yun D-J, Mengiste T (2016) Arabidopsis HOOKLESS1 Regulates Responses to Pathogens and Abscisic Acid through Interaction with MED18 and Acetylation of WRKY33 and ABI5 Chromatin. The Plant cell 28: 1662–1681
McKim SM, Koppolu R, Schnurbusch T (2018) Barley Inflorescence Architecture. In The Barley Genome, 2nd ed. Springer, Cham, Cham, pp 171–208
Pautler M, Tanaka W, Hirano H-Y, Jackson D (2013) Grass Meristems I: Shoot Apical Meristem Maintenance, Axillary Meristem Determinacy and the Floral Transition. Plant and Cell Physiology 54: 302–312
Song XJ, Kuroha T, Ayano M, Furuta T, Nagai K, Komeda N, Segami S, Miura K, Ogawa D, Kamura T, et al (2015) Rare allele of a previously unidentified histone H4 acetyltransferase enhances grain weight, yield, and plant biomass in rice. Proceedings of the National Academy of Sciences 112: 76–81
Tanaka W, Pautler M, Jackson D, Hirano H-Y (2013) Grass Meristems II: Inflorescence Architecture, Flower Development and Meristem Fate. Plant and Cell Physiology 54: 313–324
Wang L, Sun S, Jin J, Fu D, Yang X, Weng X, Xu C, Li X, Xiao J, Zhang Q (2015) Coordinated regulation of vegetative and reproductive branching in rice. Proceedings of the National Academy of Sciences 112: 15504–15509
Whipple, CJ (2017) Grass inflorescence architecture and evolution: the origin of novel signaling centers. New Phytologist 216: 367-372
Wu G, Park MY, Conway SR, Wang J-W, Weigel D, Poethig RS (2009) The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 138: 750–759