The transcription factor bZIP60 modulates the heat shock response in maize (Plant Cell)
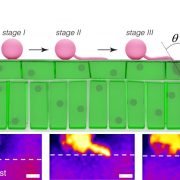
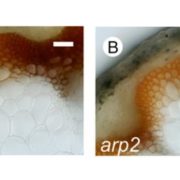
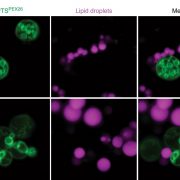
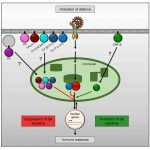
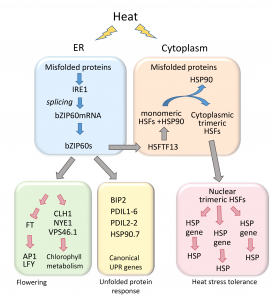 During heat stress, heat shock transcription factors upregulate heat shock protein genes and give rise to the heat shock response (HSR) that occurs in the cytoplasm, while in the endoplasmic reticulum (ER), the unfolded protein response (UPR) is also activated during heat stress. Both HSR and UPR cause the accumulation of misfolded proteins, a condition in the ER known as ER stress. Li et al. investigated the role of the Basic Leucine Zipper 60 (bZIP60) transcription factor, which plays an important role in UPR during heat stress and has previously been shown to be active following heat-induced splicing by an ER-membrane associated splicing factor. During elevated temperature, from 31ᵒC to 37ᵒC, the spliced form of bZIP60 and other HSR- and UPR-induced genes are gradually upregulated along with an increase in autophagy. bZIP60 activates the expression of HSFTF13, a type A heat shock factor, and this activation depends on the UPR-cis element in the promoter of this gene. The heat-induced “pale leaves” phenotype and senescence of the bzip60 maize mutant likely corresponds to the decreased expression of chlorophyll synthesis genes and increased expression of autophagy genes. Overall, bZIP60 mRNA could be a reliable biomarker for the UPR, and provides a link between HSR and UPR. (Summary by Min May Wong @wongminmay) Plant Cell 10.1105/tpc.20.00260
During heat stress, heat shock transcription factors upregulate heat shock protein genes and give rise to the heat shock response (HSR) that occurs in the cytoplasm, while in the endoplasmic reticulum (ER), the unfolded protein response (UPR) is also activated during heat stress. Both HSR and UPR cause the accumulation of misfolded proteins, a condition in the ER known as ER stress. Li et al. investigated the role of the Basic Leucine Zipper 60 (bZIP60) transcription factor, which plays an important role in UPR during heat stress and has previously been shown to be active following heat-induced splicing by an ER-membrane associated splicing factor. During elevated temperature, from 31ᵒC to 37ᵒC, the spliced form of bZIP60 and other HSR- and UPR-induced genes are gradually upregulated along with an increase in autophagy. bZIP60 activates the expression of HSFTF13, a type A heat shock factor, and this activation depends on the UPR-cis element in the promoter of this gene. The heat-induced “pale leaves” phenotype and senescence of the bzip60 maize mutant likely corresponds to the decreased expression of chlorophyll synthesis genes and increased expression of autophagy genes. Overall, bZIP60 mRNA could be a reliable biomarker for the UPR, and provides a link between HSR and UPR. (Summary by Min May Wong @wongminmay) Plant Cell 10.1105/tpc.20.00260