The G protein β subunit, AGB1, interacts with FERONIA in RALF1-regulated stomatal movement
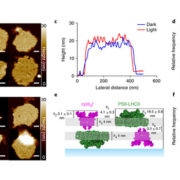
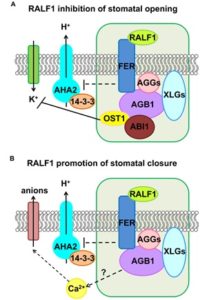 Plant Physiol. Heterotrimeric guanine nucleotide-binding (G) proteins participate in numerous processes including the regulation of hormonal responses and environmental stress. G proteins are composed of three subunits: Gα, Gβ, and Gγ. AGB1, a Gβ protein, forms a non-covalent dimer with a Gγ subunit, AGG. This AGB1-AGG dimer binds the Gα subunit GPA1. Yu et al. identified FERONIA (FER) as an interacting partner of AGB1, dependent on the presence of AGGs. FER is a receptor-like kinase (RLK) which acts as a receptor for RALF peptides. Exogenous application of RALF resulted in inhibition of stomatal opening in addition to the promotion of stomatal closure, both processes dependent on the presence of functional FER receptor and AGB1. OST1, a SNF-RELATED PROTEIN KINASE involved in ABA signalling, was also shown to interact with AGB1 and be involved in RALF inhibition of stomatal closure but, interestingly, is not required for RALF-mediated promotion of stomatal closure. These data elucidate RALF1 effects on stomatal dynamics, delineating differences in RALF mediated stomatal closure vs. inhibition of stomatal opening. (Summary by Alecia Biel) Plant Physiol. 10.1104/pp.17.01277.
Plant Physiol. Heterotrimeric guanine nucleotide-binding (G) proteins participate in numerous processes including the regulation of hormonal responses and environmental stress. G proteins are composed of three subunits: Gα, Gβ, and Gγ. AGB1, a Gβ protein, forms a non-covalent dimer with a Gγ subunit, AGG. This AGB1-AGG dimer binds the Gα subunit GPA1. Yu et al. identified FERONIA (FER) as an interacting partner of AGB1, dependent on the presence of AGGs. FER is a receptor-like kinase (RLK) which acts as a receptor for RALF peptides. Exogenous application of RALF resulted in inhibition of stomatal opening in addition to the promotion of stomatal closure, both processes dependent on the presence of functional FER receptor and AGB1. OST1, a SNF-RELATED PROTEIN KINASE involved in ABA signalling, was also shown to interact with AGB1 and be involved in RALF inhibition of stomatal closure but, interestingly, is not required for RALF-mediated promotion of stomatal closure. These data elucidate RALF1 effects on stomatal dynamics, delineating differences in RALF mediated stomatal closure vs. inhibition of stomatal opening. (Summary by Alecia Biel) Plant Physiol. 10.1104/pp.17.01277.