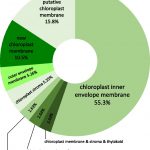
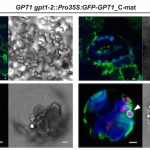
Temperature Signaling in Guard Cells
The elucidation of plant temperature signaling networks is confounded by the fact that commonly measured physiological outputs of 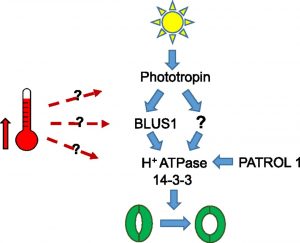 temperature change (e.g., stem elongation and flowering time) can be temporally and spatially distant from the temperature perception event, requiring intercellular, intertissue, and interorgan signaling networks. To address this constraint, Kostaki et al. (doi.org/10.1104/pp.19.01528) have used the Arabidopsis guard cell as a single-cell model to study high temperature-regulated physiological
temperature change (e.g., stem elongation and flowering time) can be temporally and spatially distant from the temperature perception event, requiring intercellular, intertissue, and interorgan signaling networks. To address this constraint, Kostaki et al. (doi.org/10.1104/pp.19.01528) have used the Arabidopsis guard cell as a single-cell model to study high temperature-regulated physiological
and gene expression responses in parallel. High temperature promotes guard cell expansion, which opens stomatal pores to facilitate leaf cooling. How the high temperature signal is perceived and transmitted to regulate stomatal aperture is unknown. By means of a reverse genetics approach, the authors reveal that high temperature-induced guard cell movement requires components involved in blue light-mediated stomatal opening, suggesting cross talk between light and temperature signaling pathways. The molecular players involved include phototropin photoreceptors, plasma membrane H+-ATPases, and multiple members of the 14-3-3 protein family. As might be predicted based on these findings, phototropin-deficient mutants display impaired rosette evapotranspiration and leaf cooling at high temperatures. Moreover, blocking the interaction of 14-3-3 proteins with their client proteins severely impairs high temperature-induced stomatal opening but has no effect on the induction of heat-sensitive guard cell transcripts. Thus, either multiple thermosensing mechanisms exist or there is a bifurcation of the thermosensory pathway upstream of plasma membrane H+-ATPase activation.