Tandem fluorescent protein timers for non-invasive relative protein lifetime measurement in plants (Plant Physiol)
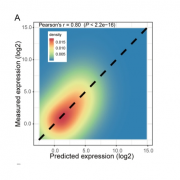
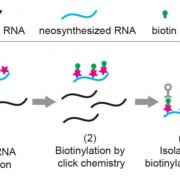
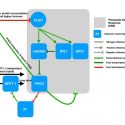
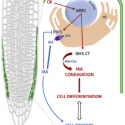
 Proteins are in a dynamic state of synthesis and degradation and their half-lives can be adjusted under various circumstances. Many rapidly degraded proteins function as regulatory molecules, such as transcription factors. The rapid turnover of these proteins is necessary to allow their levels to change quickly in response to external stimuli. Other proteins are rapidly degraded in response to specific signals, providing another mechanism for the regulation of intracellular enzyme activity. Accurate protein quantitation is essential to protein studies in a multitude of research topics. A wide array of different methods has been developed but are often unable to distinguish between protein synthesis and degradation. Therefore, specific approaches are required. Here the authors propose the tandem fluorescent protein timers (tFTs) as a powerful approach to quantify dynamic changes in plant protein stability using live imaging with high spatial and temporal resolution. In this technique two different fluorescent proteins are fused, with distinct fluorophore maturation kinetics, which allows protein age identification from the ratio of fluorescence intensities of the two fluorescent proteins. (Summarized by Francesca Resentini) Plant Physiol. 10.1104/pp.19.00051
Proteins are in a dynamic state of synthesis and degradation and their half-lives can be adjusted under various circumstances. Many rapidly degraded proteins function as regulatory molecules, such as transcription factors. The rapid turnover of these proteins is necessary to allow their levels to change quickly in response to external stimuli. Other proteins are rapidly degraded in response to specific signals, providing another mechanism for the regulation of intracellular enzyme activity. Accurate protein quantitation is essential to protein studies in a multitude of research topics. A wide array of different methods has been developed but are often unable to distinguish between protein synthesis and degradation. Therefore, specific approaches are required. Here the authors propose the tandem fluorescent protein timers (tFTs) as a powerful approach to quantify dynamic changes in plant protein stability using live imaging with high spatial and temporal resolution. In this technique two different fluorescent proteins are fused, with distinct fluorophore maturation kinetics, which allows protein age identification from the ratio of fluorescence intensities of the two fluorescent proteins. (Summarized by Francesca Resentini) Plant Physiol. 10.1104/pp.19.00051