Structural motifs of D3-D14 ubiquitin ligase in strigolactone signaling (Nature)
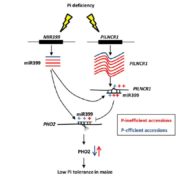
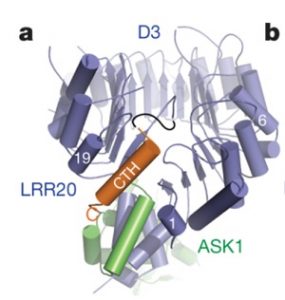 Strigalactones are phytohormones that regulate plant growth and development processes. The α/β hydrolase D14 (metabolizes strigolactone) interacts with the F-box protein D3 to ubiquitinate and degrade the transcription repressor D53. This process inhibits shoot branching. However, it is still unknown how D14 interacts with D3 to mediate hormone-dependent D53 ubiquitination. Shabek and colleagues show that the C-terminal α-helix of D3 can switch between two conformational states. One state facilitates the binding of D3 and D14 with a hydrolyzed strigolactone intermediate, while the other state recognized unmodified D14 and inhibits its enzymatic activity. This D3 motif enables D14 to recruit D53 in a strigolactone-dependent manner, which is part of the process that activates D14. This structural information helps to explain protein-based coordination between strigolactone signaling and metabolism. (Summary by Julia Miller) Nature 10.1038/s41586-018-0743-5
Strigalactones are phytohormones that regulate plant growth and development processes. The α/β hydrolase D14 (metabolizes strigolactone) interacts with the F-box protein D3 to ubiquitinate and degrade the transcription repressor D53. This process inhibits shoot branching. However, it is still unknown how D14 interacts with D3 to mediate hormone-dependent D53 ubiquitination. Shabek and colleagues show that the C-terminal α-helix of D3 can switch between two conformational states. One state facilitates the binding of D3 and D14 with a hydrolyzed strigolactone intermediate, while the other state recognized unmodified D14 and inhibits its enzymatic activity. This D3 motif enables D14 to recruit D53 in a strigolactone-dependent manner, which is part of the process that activates D14. This structural information helps to explain protein-based coordination between strigolactone signaling and metabolism. (Summary by Julia Miller) Nature 10.1038/s41586-018-0743-5