Structural and biochemical analyses of concanavalin A circular permutation (Plant Cell)
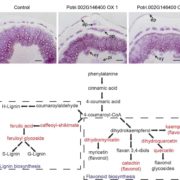
 If you have heard of concanavalin A (ConA), it probably is because it is a widely-used reagent in carbohydrate science and medical research. ConA is a lectin derived from jackbean (Canavalia ensiformis, hence the name) that binds assorted carbohydrates. It is also a fascinating protein that assembles in a unique way. Post-translationally, the pro-protein is cleaved in the middle, the front and back half sides switch and then are rejoined by the formation of a new peptide bond – this is a type of circular permutation (check out the Wikipedia page on Circular Permutation in Proteins). This sort of rearrangement usually occurs at the genetic level, or by fusion of two different proteins; ConA is unique in that it occurs post-translationally to a single polypeptide. Here, Nonis et al. expressed recombinant pro-ConA and the enzyme that cuts and pastes it, asparaginyl endopeptidase (CeAEP1), and solved crystal structures for the pro-ConA, ConA, and CeAEP1. Their analysis shows that the rearrangement leads to greater protein stability. This work is more than an investigation into an evolutionary curiosity, it also provides insights that can be applied to protein engineering. (Summary by Mary Williams @PlantTeaching) Plant Cell 10.1093/plcell/koab130
If you have heard of concanavalin A (ConA), it probably is because it is a widely-used reagent in carbohydrate science and medical research. ConA is a lectin derived from jackbean (Canavalia ensiformis, hence the name) that binds assorted carbohydrates. It is also a fascinating protein that assembles in a unique way. Post-translationally, the pro-protein is cleaved in the middle, the front and back half sides switch and then are rejoined by the formation of a new peptide bond – this is a type of circular permutation (check out the Wikipedia page on Circular Permutation in Proteins). This sort of rearrangement usually occurs at the genetic level, or by fusion of two different proteins; ConA is unique in that it occurs post-translationally to a single polypeptide. Here, Nonis et al. expressed recombinant pro-ConA and the enzyme that cuts and pastes it, asparaginyl endopeptidase (CeAEP1), and solved crystal structures for the pro-ConA, ConA, and CeAEP1. Their analysis shows that the rearrangement leads to greater protein stability. This work is more than an investigation into an evolutionary curiosity, it also provides insights that can be applied to protein engineering. (Summary by Mary Williams @PlantTeaching) Plant Cell 10.1093/plcell/koab130