Sphingolipids and Plasmodesmata
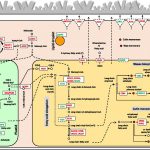
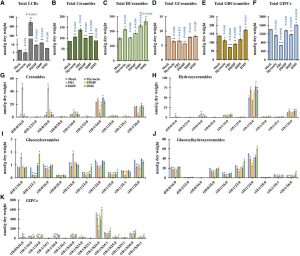 Plasmodesmata are cytoplasmic channels that physically connect the cytoplasm and endoplasmic reticulum (ER) of adjoining plant cells. These intercellular channels play important roles during plant development by allowing the molecular exchange of signaling molecules such as transcription factors, RNAs, and growth regulators. Interestingly, the plasma membrane (PM) of plasmodesmata is distinct from the general cellular PM and is enriched in sterol and sphingolipid (SL) species. The domains enriched with these special lipids are referred to as lipid rafts. The perturbation of sterol biosynthesis affects plasmodesmatal connectivity by changing the subcellular localization of two glycosylphosphatidylinositol (GPI)-anchored PD proteins, namely plasmodesmata callose binding-1 (PDCB1) and plasmodesmata-associated b-1,3-glucanase (PdBG2); this, in turn, results in the accumulation of plasmodesmata-associated callose with the consequence that the exclusion limit of plasmodesmata is lowered. Iswanto et al. (10.1104/pp.20.00401) now turn their attention to the other class of lipids enriched in the PMs of plasmodesmata, the SLs. By way of SL pathway inhibitors and two distinct SL pathway mutants, the authors report that they were able to modulate callose deposition to control symplasmic connectivity through perturbations of SL metabolism. The alteration of glucosylhydroxyceramide levels in particular disturbed the secretory machinery for the GPI-anchored PdBG2 protein, resulting in an overaccumulation of callose. In summary, this study reveals that changes in the composition of SL species and related compounds are important for the regulation of plasmodesmatal transport via their effects on PdBG2 and callose.
Plasmodesmata are cytoplasmic channels that physically connect the cytoplasm and endoplasmic reticulum (ER) of adjoining plant cells. These intercellular channels play important roles during plant development by allowing the molecular exchange of signaling molecules such as transcription factors, RNAs, and growth regulators. Interestingly, the plasma membrane (PM) of plasmodesmata is distinct from the general cellular PM and is enriched in sterol and sphingolipid (SL) species. The domains enriched with these special lipids are referred to as lipid rafts. The perturbation of sterol biosynthesis affects plasmodesmatal connectivity by changing the subcellular localization of two glycosylphosphatidylinositol (GPI)-anchored PD proteins, namely plasmodesmata callose binding-1 (PDCB1) and plasmodesmata-associated b-1,3-glucanase (PdBG2); this, in turn, results in the accumulation of plasmodesmata-associated callose with the consequence that the exclusion limit of plasmodesmata is lowered. Iswanto et al. (10.1104/pp.20.00401) now turn their attention to the other class of lipids enriched in the PMs of plasmodesmata, the SLs. By way of SL pathway inhibitors and two distinct SL pathway mutants, the authors report that they were able to modulate callose deposition to control symplasmic connectivity through perturbations of SL metabolism. The alteration of glucosylhydroxyceramide levels in particular disturbed the secretory machinery for the GPI-anchored PdBG2 protein, resulting in an overaccumulation of callose. In summary, this study reveals that changes in the composition of SL species and related compounds are important for the regulation of plasmodesmatal transport via their effects on PdBG2 and callose.