Special issue: Orchestrating the proteome with post-translational modifications (J. Exp. Bot.)
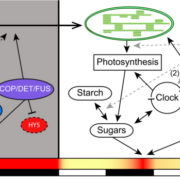
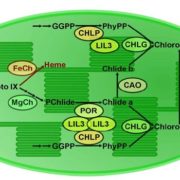
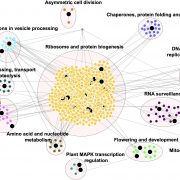
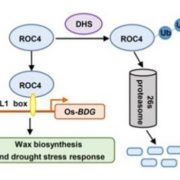
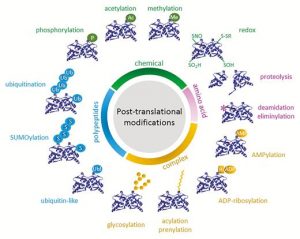 I guess we’re well past the stage of thinking “one gene – one protein”, but even a single polypeptide isn’t really one protein, due to the huge number of different types of post-translational modification (PTM) it can be subjected to. These are summarized in the illustration that accompanies the editorial by Spoel (10.1093/jxb/ery295) of this special issue of J. Exp. Bot. PTMs can be chemical (phosphorylation, acetylation, redox), involve ubiquitination or SUMOylation, involve changes in primary sequence (proteolysis, deamidation), or other, including glycosylation and prenylation. Some PTMs are reversible, others irreversible, and most are mediated by sets of enzymes called writers, readers and erasers that add, interact with or remove these PTMs. Consider this: “For example, in Arabidopsis phosphorylation is regulated by more than 1100 genes encoding protein kinases and phosphatases, whereas over 1600 genes encode ubiquitin ligases and deubiquitinases.” The papers colletected in this special issue span from basic to applied, and highlight how rapidly our understanding is expanding thanks to several new experimental technologies. (Summary by Mary Williams) J. Exp. Bot. Volume 69, Issue 19, 31 August 2018
I guess we’re well past the stage of thinking “one gene – one protein”, but even a single polypeptide isn’t really one protein, due to the huge number of different types of post-translational modification (PTM) it can be subjected to. These are summarized in the illustration that accompanies the editorial by Spoel (10.1093/jxb/ery295) of this special issue of J. Exp. Bot. PTMs can be chemical (phosphorylation, acetylation, redox), involve ubiquitination or SUMOylation, involve changes in primary sequence (proteolysis, deamidation), or other, including glycosylation and prenylation. Some PTMs are reversible, others irreversible, and most are mediated by sets of enzymes called writers, readers and erasers that add, interact with or remove these PTMs. Consider this: “For example, in Arabidopsis phosphorylation is regulated by more than 1100 genes encoding protein kinases and phosphatases, whereas over 1600 genes encode ubiquitin ligases and deubiquitinases.” The papers colletected in this special issue span from basic to applied, and highlight how rapidly our understanding is expanding thanks to several new experimental technologies. (Summary by Mary Williams) J. Exp. Bot. Volume 69, Issue 19, 31 August 2018