Spatial resolution of an integrated C4+CAM photosynthetic metabolism (Sci. Advances)
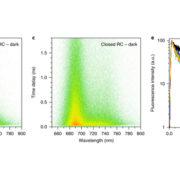
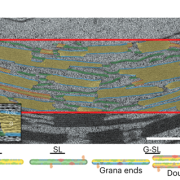
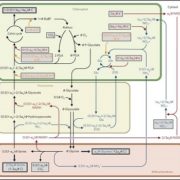
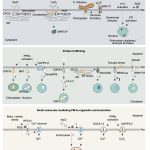
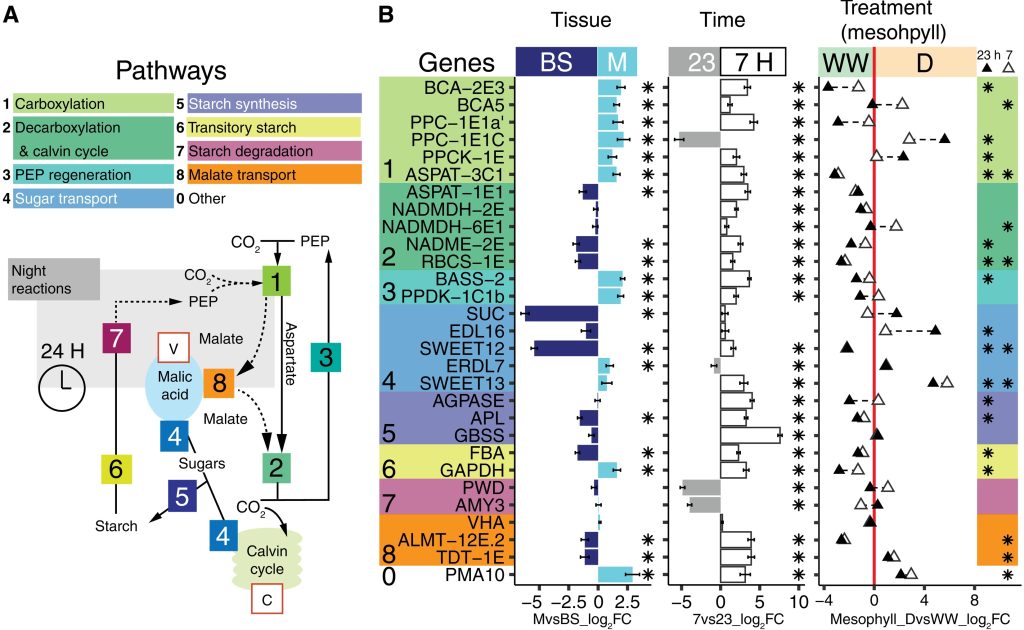 C4 and CAM are both photosynthetic strategies that concentrate CO2 upstream of RuBisCO, somewhat uncoupling photosynthetic carbon fixation from transpirational water loss. Both strategies use the enzyme PEP-carboxylase to produce an organic acid, which can then be decarboxylated to provide CO2 to RuBisCO. In the C4 system, PEP-carboxylase operates in mesophyll cells and RuBisCO in bundle sheath cells, with the organic acid shuttling between them as a CO2 carrier. Rather than this physical separation, CAM plants separate their carboxylases by time; CO2 uptake occurs at night when transpiration is lower, it is stored as organic acids in the vacuole, and then released by decarboxylation in daylight when RuBisCO is active. Moreno-Villena et al. explored the carbon-concentrating mechanisms of Portulaca oleracea, a C4 species that also carries out CAM under drought conditions. They carried out RNA sequencing on isolated mesophyll and bundle sheath cells from well-watered and droughted plants. Their first surprise was that genes involved in both C4 and CAM were expressed in the same bundle sheath cells, arguing against a previous assumption that these reactions took place in spatial isolation. The second finding was that the two pathways are actually linked. This finding comes from a metabolic model as well as flux balance analysis. Under drought conditions, CAM-produced malate feeds into the C4 decarboxylation cycle and final CO2 assimilation. The authors then speculate on why this efficient coupling of C4 and CAM is apparently rare, suggesting that Portulaca was most likely an ancestrally C3+CAM species that evolved C4. They also observe that as fast-growing, self-compatible plants, Portulaca is amenable to development as a model system, and that further study could lead to the incorporation of CAM into C4 crops, thereby increasing their drought tolerance without sacrificing productivity. (Summary by Mary Williams @PlantTeaching) Sci. Advances 10.1126/sciadv.abn2349
C4 and CAM are both photosynthetic strategies that concentrate CO2 upstream of RuBisCO, somewhat uncoupling photosynthetic carbon fixation from transpirational water loss. Both strategies use the enzyme PEP-carboxylase to produce an organic acid, which can then be decarboxylated to provide CO2 to RuBisCO. In the C4 system, PEP-carboxylase operates in mesophyll cells and RuBisCO in bundle sheath cells, with the organic acid shuttling between them as a CO2 carrier. Rather than this physical separation, CAM plants separate their carboxylases by time; CO2 uptake occurs at night when transpiration is lower, it is stored as organic acids in the vacuole, and then released by decarboxylation in daylight when RuBisCO is active. Moreno-Villena et al. explored the carbon-concentrating mechanisms of Portulaca oleracea, a C4 species that also carries out CAM under drought conditions. They carried out RNA sequencing on isolated mesophyll and bundle sheath cells from well-watered and droughted plants. Their first surprise was that genes involved in both C4 and CAM were expressed in the same bundle sheath cells, arguing against a previous assumption that these reactions took place in spatial isolation. The second finding was that the two pathways are actually linked. This finding comes from a metabolic model as well as flux balance analysis. Under drought conditions, CAM-produced malate feeds into the C4 decarboxylation cycle and final CO2 assimilation. The authors then speculate on why this efficient coupling of C4 and CAM is apparently rare, suggesting that Portulaca was most likely an ancestrally C3+CAM species that evolved C4. They also observe that as fast-growing, self-compatible plants, Portulaca is amenable to development as a model system, and that further study could lead to the incorporation of CAM into C4 crops, thereby increasing their drought tolerance without sacrificing productivity. (Summary by Mary Williams @PlantTeaching) Sci. Advances 10.1126/sciadv.abn2349