Shape-Shifters: How Strigolactone Signaling Helps Shape the Shoot
IN BRIEF by Jennifer Lockhart [email protected]
When a deer eats the primary shoot of a plant, this can activate a nearby dormant axillary bud, causing it to form a secondary shoot. Genetic and environmental factors also affect shoot architecture, which strongly influences crop productivity. Changes in shoot architecture are mediated by long-distance signals, including phytohormones. For example, strigolactones (SLs) help regulate shoot branching, as well as branching angle, plant height, stem thickness, and leaf blade elongation (reviewed in Borghi et al., 2016). In general, little is known about how downstream targets of SLs regulate shoot architecture, including whether different responses occur via different pathways.
A bit more is known about the proximal events in SL signaling. The SL receptor, the α/β-fold hydrolase DWARF14 (D14), appears to have an SL-dependent association with the F-box protein MAX2, likely forming part of a ubiquitin ligase complex that functions in SL signaling using a classic tactic: regulating target protein degradation by the 26S proteasome. The main (and perhaps only) proteolytic targets of SL signaling are SUPPRESSOR OF MORE AXILLARY GROWTH2-LIKE (SMXL) family members, such as SMXL7 in Arabidopsis thaliana. SMXL proteins contain evolutionarily conserved ETHYLENE-RESPONSE FACTOR Amphiphilic Repression (EAR) motifs, which might allow them to interact with their targets, although the underlying mechanism is unclear (reviewed in Bennett and Leyser, 2014).
Using a powerful mechanistic approach, Liang et al. (2016) provide direct, in planta evidence in support of this D14-mediated SL signaling pathway. First, they assessed the subcellular localization of SMXL7 by fusing it to a fluorescent reporter gene and expressing it in Nicotiana benthamiana epidermal cells, finding that it strongly localized to the nucleus. The nuclear localization signal in SMXL7 is required for its function; transforming smxl max2 mutants with wild-type SMXL7, but not a version lacking this signal, restored their branching patterns to wild-type levels. Similar analyses revealed that both D14 and MAX2 primarily localize to the nucleus. The interactions between these components were then explored in N. benthamiana leaf epidermal cells harboring a suite of reporter-protein constructs using Förster resonance energy transfer with fluorescence lifetime imaging microscopy. This technique can reveal whether two fluorophores are within several nanometers of each other, a distance sufficiently close for molecular interactions to occur. SMXL7 localized to speckles in the nucleus, and D14 appeared to be recruited to these speckles in response to treatment with a synthetic SL analog. This interaction led to the degradation of SMXL7, as predicted by the model. Although MAX2 interacted with D14 as well, cotransfection of these proteins did not lead to SMXL7 degradation. SMXL7 appears to interact only indirectly with MAX2, with D14 acting as a bridge to bring these proteins together.
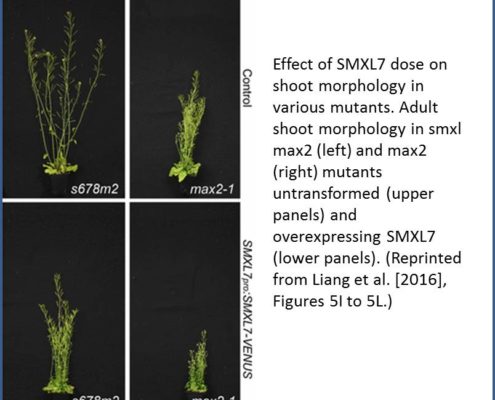
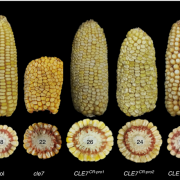
The authors then explored the downstream effects of D14-mediated SL signaling on shoot architecture by expressing SMXL7 at various levels in Arabidopsis. Overexpressing SMXL7 in wild-type plants had only minor effects on shoot architecture, whereas mutants deficient in MAX2-induced degradation of SMXL7 showed dose-dependent changes in shoot architecture in response to SMXL7 overexpression (see figure). Wild-type plants expressing stabilized SMXL7 resembled SL-deficient or SL-signaling mutants, confirming the importance of SMXL7 degradation in shoot architecture. Interestingly, transforming the plants with SMXL7 variants with a modified or deleted EAR motif had different effects on different SL-regulated shoot phenotypes. For example, in the wild-type background, removing the EAR motif had little effect on leaf morphology and branch angle, whereas branch number and plant height were highly sensitive to EAR mutation. Therefore, rather than magic spells, SMXLs might use multiple pathways to regulate different shoot architectural traits, a notion ripe for further exploration.
 REFERENCES
REFERENCES
Bennett, T., Leyser, O. (2014). Strigolactone signalling: standing on the shoulders of DWARFs. Curr. Opin. Plant Biol. 22: 7–13.
Borghi, L., Liu, G.-W., Emonet, A., Kretzschmar, T., Martinoia, E. (2016). The importance of strigolactone transport regulation for symbiotic signaling and shoot branching. Planta 243: 1351–1360.
Liang, Y., Ward, S., Li, P., Bennett, T., Leyser, O. (2016). SMAX1-LIKE7 signals from the nucleus to regulate shoot development in Arabidopsis via partially EAR motif-independent mechanisms. Plant Cell 28: 1581–1601.





Leave a Reply
Want to join the discussion?Feel free to contribute!