Senescence: the genetics behind stay-green corn
Senescence results from two ineluctable laws of nature: every living entity will eventually die and when it does, nothing will be lost, as everything will be recycled. At the organism level, senescence is an integral part of the plant life cycle, under strict age-dependent genetic control. This dismantlement of living tissues and recycling of essential elements aims to promote plant success (Schippers et al. 2015).
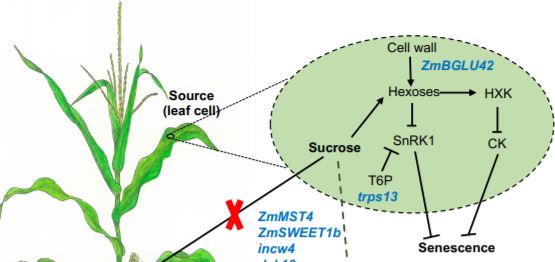
For a plant, production of viable seeds requires a large investment of nutrients, all to increase the chances of survival of the next generation. To get this energy, the plant relocates necessary nutrients from sources to sinks (Figure). However, when a plant sacrifices leaves, and therefore photosynthesis units for reproductive needs, it can ultimately cause the death of the whole plant. Delaying leaf senescence in ‘stay-green’ crops has been a beneficial strategy for plant breeding, as crop productivity and senescence are associated (Thomas & Howarth 2000). Although stay-green cultivars were developed decades ago, the genetic architecture of this trait remains poorly understood as senescence is a complex developmental program dependent on both internal and external factors.
Sekhon et al. (2019) revealed the genetic architecture of senescence in maize (Zea mays L.). They studied a stay-green line that maintained full photosynthetic capacity and grain filling for 6 critical additional days compared to a naturally senescent genotype. To reveal the genetic architecture of stay-green, the authors used a system genetics approach that integrated both gene identification by genome-wide association study (GWAS) in a US maize diversity panel (Hirsch et al. 2014), and co-expression networks derived from transcriptomes at different developmental stages.
 The system genetics framework revealed 64 candidates genes among which 48 were differentially expressed, and 30 were annotated as senescence associated genes in Arabidopsis thaliana. Overall, gene expression profiles revealed the up-regulation of autophagy as well as lipid, carbohydrate and amino acid metabolism and/or transport but down-regulation of photosynthesis and abscisic acid synthesis.
The system genetics framework revealed 64 candidates genes among which 48 were differentially expressed, and 30 were annotated as senescence associated genes in Arabidopsis thaliana. Overall, gene expression profiles revealed the up-regulation of autophagy as well as lipid, carbohydrate and amino acid metabolism and/or transport but down-regulation of photosynthesis and abscisic acid synthesis.
The candidate genes revealed the role of cell wall and biological processes such as proteolysis, and sugar signaling and transport in senescence in maize. The role of one of the candidate genes, mir3 (maize insect resistance 3), which encodes a putative cysteine protease was validated in senescence. Knockout of mir3 in Arabidopsis delayed leaf senescence, suggesting the role of this gene in senescence in both monocots and dicots.
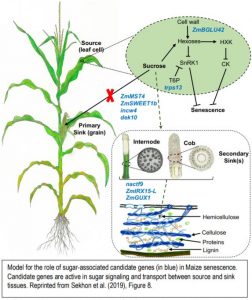
Sekhon et al 2019 also explained the role of sugar partitioning and sugar signaling in senescence by placing nine candidate genes in a model (Figure). The candidate genes represent diverse processes including sugar mediated signaling (trps13), transport (ZmSWEET1b and ZmMST4), and control of sugar uptake (dek10). Cell walls also play a key role in stay-green lines, both as a source of sugars that potentially act in senescence signaling, and as an alternative sink for excess sugars. The authors propose that the cell wall invertase (incw4) unloads sugars in the source while they are incorporated in the sink by a NAC transcription factor (nacft9) or transformed into hemicellulose by ZmIRX15-L and ZmGUX1. nacft9 is proposed to be an important regulator of delayed senescence in the stay-green lines.
Epistatic interactions among the candidate genes were also detected. Other potentially valuable candidate genes require further investigation to reveal their mechanistic involvement in senescence.
Figure legend: Model for the role of sugar-associated candidate genes (in blue) in Maize senescence. Candidate genes are active in sugar signaling and transport between source and sink tissues. Reprinted from Sekhon et al. (2019), Figure 8.
REFERENCES
Hirsch CN, Foerster JM, Johnson JM, Sekhon RS, Muttoni G, Vaillancourt B, Peñagaricano F, Lindquist E, Pedraza MA, Barry K, de Leon N. (2014). Insights into the maize pan-genome and pan-transcriptome. The Plant Cell 26:121-35.
Schippers JH, Schmidt R, Wagstaff C, Jing HC. (2015). Living to die and dying to live: the survival strategy behind leaf senescence. Plant Physiology 169: 914-930.
Sekhon RD, Saski C, Kumar R, Flinn BS, Luo F, Beissinger TM, Ackerman AJ, Breitzman MW, Bridges WC, de Leon N, Kaeppler SM. (2019) Integrated Genome-Scale Analysis identifies novel genes and networks underlying senescence in Maize. The Plant Cell https://doi.org/10.1105/tpc.18.00930
Thomas H, & Howarth CJ. (2000). Five ways to stay green. Journal of Experimental Botany 51: 329-337.