Rubisco condensate formation by CcmM in β-carboxysome biogenesis (Nature) ($)
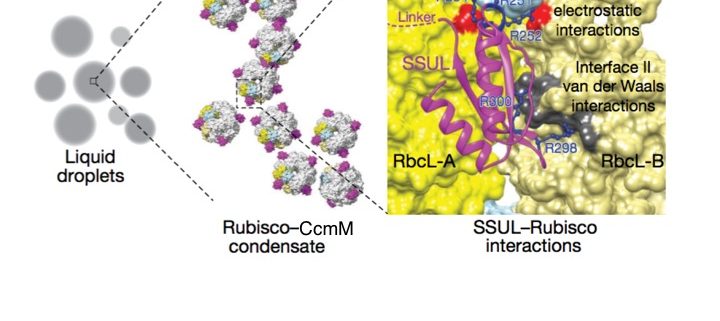
Cyanobacterial carbon-dioxide concentrating mechanisms elevate intracellular inorganic carbon as bicarbonate, and then concentrate it as carbon-dioxide around the enzyme Rubisco in specialized protein micro-compartments called carboxysomes. The formation of B-carboxysomes involves an aggregation between Rubisco and the major scaffolding protein of the carboxysome, called CcmM. CcmM is composed of several repeat modules that can resemble the small subunit of Rubisco, and it was long assumed that they replaced these small subunits of Rubisco when the proteins interact, linking Rubisco proteins within the carboxysome. However, the highly dynamic complex formed between CcmM and Rubisco has made it difficult to determine the structural formation by traditional structural biology methods. Wang et al. used purified Rubisco and CcmM from Synechococcus elongates to explore the structural relationship between these two proteins, and unexpectedly found that they form a spontaneous aggregate, via liquid-like phase separation. Using the turbidity of this condensate and variants of the CcmM and Rubisco proteins, they were able to determine the regions of importance, and then resolved the structure basis of the interaction using cryo-electron microscopy to find that the small-subunit like modules of the CcmM bind close to the equatorial region of Rubisco between large subunit dimers, but also interact with the Rubisco small subunit, ensuring that only the intact holoenzyme is incorporated into the B-carboxysome. (Summary by Amanda Cavanagh) Nature 10.1038/s41586-019-0880-5