Roots Calling Leaves: Oxylipins in Induced Systemic Resistance
Wang et al. identify signals related to induced systemic resistance produced by a root-colonizing fungus. Plant Cell https://doi.org/10.1105/tpc.19.00487
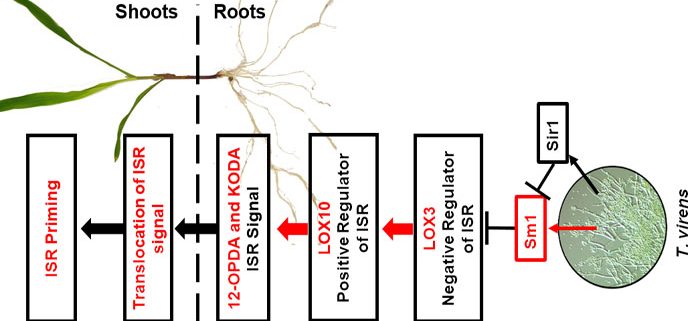
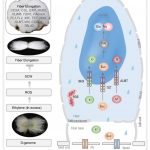
Background: Trichoderma virens is an agriculturally-relevant root-colonizing fungus that provides its plant hosts many benefits, such as increased growth, destruction of soil-borne pathogens, and enhanced systemic resistance against foliar pathogens, termed induced systemic resistance (ISR). The mobile ISR signals are not known, though it is postulated to involve phytohormones jasmonic acid (JA) and ethylene. JA is one of hundreds of oxylipins, which are hypothesized to regulate many physiological processes including ISR. Lipoxygenases (LOX) are key enzymes in the biosynthesis of oxylipins. Previously, we identified maize root-expressed LOX3 as a negative regulator of ISR, because lox3 knock-out mutants displayed strong constitutive ISR. Additionally, we identified T. virens mutants that either cannot trigger ISR or trigger a stronger systemic response.
Question: We hypothesized that signals other than JA are responsible for ISR, which originate in the roots and are transported through the plant vasculature. By utilizing ISR-positive and -negative mutants of both maize (Zea mays) and T. virens, we profiled xylem sap of T. virens-treated maize to identify potential ISR signals.
Findings: Here, LOX10 was identified as a positive regulator of ISR, as instead of conferring ISR, T. virens-colonized lox10 mutants became more susceptible to leaf pathogens. Metabolite profiling was performed on xylem saps collected from lox3 and lox10 mutants treated with ISR-positive or ISR-negative strains of T. virens. The screening revealed that accumulation of two oxylipins, the JA precursor, 12-OPDA, and α-ketol, KODA, correlated with ISR activation. Sap transfusion of either of these two compounds enhanced ISR of receiver plants against infection in a dose-dependent manner. Surprisingly, transfusion with JA increased susceptibility to infection. Furthermore, JA-deficient mutants still benefited from T. virens-triggered ISR.
Next steps: While 12-OPDA biosynthesis is well understood, specific maize enzymes for KODA biosynthesis are unknown. Our future work will focus on screening available maize mutants to identify enzymes responsible for KODA biosynthesis. It will also be of interest to identify receptors for 12-OPDA and KODA that, when bound, trigger ISR.
Ken-Der Wang, Eli J. Borrego, Charles M. Kenerley, Mike V Kolomiets (2019) Oxylipins Other Than Jasmonic Acid Are Xylem-Resident Signals Regulating Systemic Resistance Induced by Trichoderma virens In Maize. https://doi.org/10.1105/tpc.19.00487