Remodeling Flowering: CHROMATIN REMODELING 4 Promotes the Floral Transition
 Plants that fail to flower at the right time will lose out on seed production. Hence, the transition from vegetative growth to the reproductive phase depends strongly on environmental signals. Cold winter temperatures and long days are seasonal cues that stimulate flowering through vernalization and photoperiodic pathways, whereas high ambient temperatures also promote floral transition (Srikanth and Schmid, 2011). As endogenous signals, high levels of gibberellic acids (GAs) (Mutasa-Göttgens and Hedden, 2009) and increasing plant age (Wang, 2014) lead to flowering in the absence of environmental triggers. In this issue, Sang et al. (2020) took an elegant approach to identify genes that act in the endogenous pathways for floral transition. They constructed a quintuple mutant deficient in induction of flowering by environmental signals and mutagenized it further to screen for endogenous regulators of flowering, identifying CHROMATIN REMODELING 4 (CHR4) as a positive regulator of floral transition.
Plants that fail to flower at the right time will lose out on seed production. Hence, the transition from vegetative growth to the reproductive phase depends strongly on environmental signals. Cold winter temperatures and long days are seasonal cues that stimulate flowering through vernalization and photoperiodic pathways, whereas high ambient temperatures also promote floral transition (Srikanth and Schmid, 2011). As endogenous signals, high levels of gibberellic acids (GAs) (Mutasa-Göttgens and Hedden, 2009) and increasing plant age (Wang, 2014) lead to flowering in the absence of environmental triggers. In this issue, Sang et al. (2020) took an elegant approach to identify genes that act in the endogenous pathways for floral transition. They constructed a quintuple mutant deficient in induction of flowering by environmental signals and mutagenized it further to screen for endogenous regulators of flowering, identifying CHROMATIN REMODELING 4 (CHR4) as a positive regulator of floral transition.
The quintuple mutant svp-41 flc-3 ft-10 tsf-1 soc1-2 had impaired flowering responses to long day conditions, high temperature, and GA treatment, indicating that floral transition occurs via endogenous signals in these plants. RNA sequencing from samples of shoot apices at different time points after sowing showed higher mRNA levels of known endogenous flowering regulators in the quintuple mutant, such as SQUAMOSA PROMOTER BINDING PROTEIN-LIKEs (SPLs) that promote flowering through the age- and GA-dependent pathways. Mutagenesis of the quintuple mutant and subsequent screening for late flowering led to the identification of two mutant lines named qem1 and qem2.
Fast-isogenic mapping and complementation analysis showed that the late-flowering phenotype in qem1 was caused by a mutation in the GA20ox2 gene that is involved in GA biosynthesis. Together with the higher GA20ox2 and SPLs mRNA levels in the quintuple mutant compared to control plants, these data suggest that the GA pathway is involved in the early flowering of the quintuple mutant.
The mutation causing late flowering of qem2 was localized to CHR4, so the authors analyzed the phenotypes of qem2 side by side with the quintuple mutant and compared the chr4-2 mutant with respective wild-type Col-0. Plants deficient in CHR4 function had faster leaf production in later rosette growth stages, more cauline leaves, and larger shoot apical meristems, whereas all these phenotypes were stronger in the quintuple mutant background. Plants with the chr4 mutant alleles bolted earlier but took longer to flower after bolting. The authors suggest that the accelerated transition from vegetative phase to bolting in chr4 mutants could be caused by higher SPL15 expression levels. Both in qem2 and chr4-2, formation of the floral primordia was delayed, supporting an important role for CHR4 in establishing floral meristem identity.
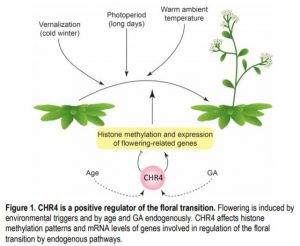
CHR4-interacting proteins were identified by immunoprecipitation and included several transcription factors of the MADS, SPL, and AP2 families that regulate the floral transition and floral meristem identity, linking CHR4 to known regulators of flowering. Plants with chr4 alleles had alterations in mRNA levels of several genes involved in floral transition, but also had different patterns of histone methylation. Genomic regions with increased or reduced levels of histone methylation were found in chr4, and were associated with known floral regulators such as miR156D and SPL15 that affect flowering in the age pathway and with genes involved in hormonal regulation through GAs, auxins and cytokinins. The changed histone modification patterns and expression levels of components of the endogenous GA- and age-dependent pathways of floral induction, such as different SPLs, and the various interaction partners of CHR4 suggest that CHR4 promotes flowering through several complexes and pathways (see figure). A further challenge lies in understanding how CHR4 achieves its manifold effects on gene expression.
Hanna Hõrak
Institute of Technology, University of Tartu, Estonia
ORCID: 0000-0002-6392-859X
REFERENCES
Mutasa-Göttgens, E. and Hedden, P. (2009). Gibberellin as a factor in floral regulatory networks. J. Exp. Bot. 60: 1979–1989.
Sang, Q., Pajoro, A., Sun, H., Song, B., Yang, X., Stolze, S.-C., Andrés, F., Schneeberger, K., Nakagami, H. and Coupland, G. (2020). Mutagenesis of a Quintuple Mutant Impaired in Environmental Responses Reveals Roles for CHROMATIN REMODELING 4 in Arabidopsis Floral Transition. Published March 2020. DOI: https://doi.org/10.1105/tpc.19.00992
Srikanth, A. and Schmid, M. (2011). Regulation of flowering time: all roads lead to Rome. Cell. Mol. Life Sci. 68: 2013–2037.
Wang, J.-W. (2014). Regulation of flowering time by the miR156-mediated age pathway. J. Exp. Bot. 65: 4723–4730.