Redox regulation of NADP-malate dehydrogenase is vital under fluctuating light environment (PNAS)
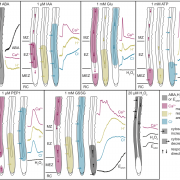
 Redox switches regulate photosynthetic reactions and are mediated by conserved cysteine pairs, activated by reduction under light and deactivated by oxidation in the dark. Those reactions are mediated by thioredoxins (Trx). Usually, only plastidic and not cytosolic isozymes show these redox switches. As an example, the redox-regulated enzyme malate dehydrogenase (MDH) produces malate using NADPH to export reducing products from chloroplasts to the cytosol, which balances the ATP/NADPH ratio in the chloroplast. The activity of MDH in the dark is strictly regulated by two switches at the N and C terminus, suggesting that complete deactivation is necessary to generate acclimation responses. Yokochi et al. used CRISPR-Cas9 to generate mutant plants (MDHΔC-CR) with deleted thiol-based redox switch. The mutants showed no phenotypic changes under standard long-day conditions. However, under short-day or fluctuating light the mutants showed a stunted growth phenotype. The metabolite dynamics in the mutants indicated that the oxidized NADP pool and ferredoxin, which serve as electron acceptors for PSI, are increased in MDHΔC-CR. In conclusion, the thiol-switch redox regulation of MDH was found to be required to maintain plastidic NADPH homeostasis under fluctuating light conditions. (Summary by Camila Ribeiro @camicribeiro86) Proc. Natl. Acad. Sci. USA 10.1073/pnas.2016903118.
Redox switches regulate photosynthetic reactions and are mediated by conserved cysteine pairs, activated by reduction under light and deactivated by oxidation in the dark. Those reactions are mediated by thioredoxins (Trx). Usually, only plastidic and not cytosolic isozymes show these redox switches. As an example, the redox-regulated enzyme malate dehydrogenase (MDH) produces malate using NADPH to export reducing products from chloroplasts to the cytosol, which balances the ATP/NADPH ratio in the chloroplast. The activity of MDH in the dark is strictly regulated by two switches at the N and C terminus, suggesting that complete deactivation is necessary to generate acclimation responses. Yokochi et al. used CRISPR-Cas9 to generate mutant plants (MDHΔC-CR) with deleted thiol-based redox switch. The mutants showed no phenotypic changes under standard long-day conditions. However, under short-day or fluctuating light the mutants showed a stunted growth phenotype. The metabolite dynamics in the mutants indicated that the oxidized NADP pool and ferredoxin, which serve as electron acceptors for PSI, are increased in MDHΔC-CR. In conclusion, the thiol-switch redox regulation of MDH was found to be required to maintain plastidic NADPH homeostasis under fluctuating light conditions. (Summary by Camila Ribeiro @camicribeiro86) Proc. Natl. Acad. Sci. USA 10.1073/pnas.2016903118.